Put the lid back, quickly insert the thermometer and stirring rod, and stir the mixture vigorously. on behalf of the United States of America. endothermic which was apparent by the negative T value. Therefore it is recommended as being more suitable as a teacher demonstration. The calculation is based on the calorimetry equation: Heat given off or absorbed = (mass)(specific heat)(change in temperature). Because Hsoln depends on the concentration of the solute, diluting a solution can produce a change in enthalpy. Enthalpy is a state function whose change indicates the amount of heat transferred from a system to its surroundings or vice versa, at constant pressure. 33.2C C. 21.5C D. 13.5C A What is the heat of solution (q) for the neutralization reaction between NH and HCl? Now that we got the basics cleared away, let us look at your problem. Record the temperature of the water at 30 second intervals for 10 minutes until the temperature becomes constant. It works as a flux by cleaning the surface of workpieces by reacting with the metal oxides at the surface to form a volatile metal chloride. Obtain two Styrofoam cups. He then observes the temperature of the water fall from 21.0 C to 17.4 C over the course of 3.6 minutes. Cross), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Give Me Liberty! Practical Chemistry activities accompanyPractical PhysicsandPractical Biology. The mixture becomes slushy as a liquid is formed, together with a white suspension. Data compilation copyright displays seen below. Yes, the dissolution of ammonium chloride in water is endothermic. H = standard enthalpy (kJ/mol) From the values above they could also be asked to calculate the actual entropy change for the system and the surroundings, and hence Stotalor G for the reaction and confirm that the process is spontaneous. answered 05/12/21, Ph.D. University Professor with 10+ years Tutoring Experience. Ammonium chloride reacts with a strong base, like sodium hydroxide, to release ammonia gas: Similarly, ammonium chloride also reacts with alkali-metal carbonates at elevated temperatures, giving ammonia and alkali-metal chloride: A solution of 5% by mass of ammonium chloride in water has a pH in the range 4.6 to 6.0.[12]. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Calculate the heat of solution of CaCl2(Hsoln) in kJ/mol. This is a question from a lab previously done for my chem class. WebThis means that the heat energy will flow from the water to the ammonium chloride. The earliest mention of ammonium chloride was in 554 in China. Determine theheat of solution of two ionic compounds: ammonium chloride and calcium chloride. The calculation is Calculate the mass of the solution [(b)+(d)(a)], in grams. In general, for dissolving salts in solution, this is the case. The reaction of Ammonium chloride with water: (a) an acid is added to a basic solution. Good Question, always remember that you cannot, in most situations, physically measure the change in the system. Observe chemical changes in this microscale experiment with a spooky twist. Why is the enthalpy of formation of oxygen zero? Consider chemical reactions in terms of energy, using the terms exothermic, endothermic and activation energy, and use simple energy profile diagrams to illustrate energy changes. It had a secondary use to provide white smoke, but its ready double decomposition reaction with potassium chlorate producing the highly unstable ammonium chlorate made its use very dangerous.[14][15][16]. The final experiment explored the solubility of NH4Cl, and its enthalpy of dissolution was found to endothermic which was apparent by the negative T value. Ammonium chloride is used as an expectorant in cough medicine. Improving the copy in the close modal and post notices - 2023 edition, Entropy and enthalpy change on mixing liquids. If the salt is CaCl 2, heat is released to produce a solution with a temperature of about 90C; hence the product is an instant hot compress. If the salt is Learn more about Stack Overflow the company, and our products. The official H dissolution for NH4CL was 14780 J/mol at rtp, the experimental value was 11750 J/mol. 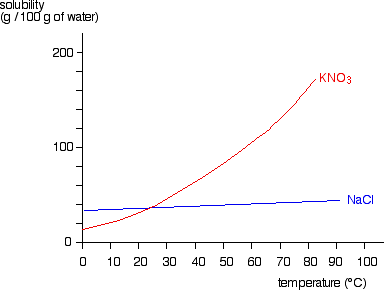
 Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. The system in this case, consists of the individual ions that separate when placed in solution ($\ce{NH_4^{+}}$ and $\ce{Cl^{-}}$). Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? According to the invention, acrylamide, 2-acrylamido-2-methyl propanesulfonic acid, and polyvinyl pyrrolidone are dissolved into a mixed water solution; a temperature is increased to 30-40 DEG C, and dmethyl diallyl The reaction of Ammonium chloride with water: Asking for help, clarification, or responding to other answers. This is the initial temperature (Ti). Exam 1 Review - Study material taken from the professors slides and the textbook. The amount of heat released or absorbed when a substance is dissolved is not a constant; it depends on the final concentration of the solute. A bag of concentrated sodium acetate solution can be carried until heat is needed, at which time vigorous agitation induces crystallization and heat is released. This is a resource from thePractical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Calculate the number of moles of calcium chloride used. Why is HCl called Hydrogen Chloride (by IUPAC naming)? Information about your use of this website will be shared with Google and other third parties. Around the turn of the 20th century, ammonium chloride was used in aqueous solution as the electrolyte in Leclanch cells that found a commercial use as the "local battery" in subscribers' telephone installations. and enthalpy of dissolution (J/mol) were determined. You are using an out of date browser. Determine whichdissolutionprocess is endothermic and which one is exothermic. 2. 34 C. and Informatics, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data), https://doi.org/10.1016/S0021-9614(71)80036-8, Enthalpy of formation of solid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of sublimation at standard conditions. NIST Standard Reference In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Is sign convention for delta H and enthapy change of a solution different? t = temperature (K) / 1000. Future versions of this site may rely on
6. My reasoning: if the temperature of the solution decreases, it means heat was released into the surroundings, so the enthalpy change is negative. Even though I submitted with a different response, at least I know what the real deal is. Data Program, but require an annual fee to access. Although the experiment can be safely carried out as a class experiment (with GCSE or A-level candidates in mind), it lasts only about 5 minutes and may not be worth the extra time spent by students setting up and clearing away. Now, I understand there is an endothermic reaction and it is caused by the lattice All rights reserved.
7.
Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. The system in this case, consists of the individual ions that separate when placed in solution ($\ce{NH_4^{+}}$ and $\ce{Cl^{-}}$). Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? According to the invention, acrylamide, 2-acrylamido-2-methyl propanesulfonic acid, and polyvinyl pyrrolidone are dissolved into a mixed water solution; a temperature is increased to 30-40 DEG C, and dmethyl diallyl The reaction of Ammonium chloride with water: Asking for help, clarification, or responding to other answers. This is the initial temperature (Ti). Exam 1 Review - Study material taken from the professors slides and the textbook. The amount of heat released or absorbed when a substance is dissolved is not a constant; it depends on the final concentration of the solute. A bag of concentrated sodium acetate solution can be carried until heat is needed, at which time vigorous agitation induces crystallization and heat is released. This is a resource from thePractical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Calculate the number of moles of calcium chloride used. Why is HCl called Hydrogen Chloride (by IUPAC naming)? Information about your use of this website will be shared with Google and other third parties. Around the turn of the 20th century, ammonium chloride was used in aqueous solution as the electrolyte in Leclanch cells that found a commercial use as the "local battery" in subscribers' telephone installations. and enthalpy of dissolution (J/mol) were determined. You are using an out of date browser. Determine whichdissolutionprocess is endothermic and which one is exothermic. 2. 34 C. and Informatics, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data), https://doi.org/10.1016/S0021-9614(71)80036-8, Enthalpy of formation of solid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of sublimation at standard conditions. NIST Standard Reference In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Is sign convention for delta H and enthapy change of a solution different? t = temperature (K) / 1000. Future versions of this site may rely on
6. My reasoning: if the temperature of the solution decreases, it means heat was released into the surroundings, so the enthalpy change is negative. Even though I submitted with a different response, at least I know what the real deal is. Data Program, but require an annual fee to access. Although the experiment can be safely carried out as a class experiment (with GCSE or A-level candidates in mind), it lasts only about 5 minutes and may not be worth the extra time spent by students setting up and clearing away. Now, I understand there is an endothermic reaction and it is caused by the lattice All rights reserved.
7.  This means that the temperature of the system increases. How will you explain the difference in your values? A process that gives off heat is called exothermic(-Hsoln), and a process that absorbs heat is called endothermic(+Hsoln). In its naturally occurring mineralogic form, it is known as sal ammoniac. Soc., Dalton Trans., 1972, 1943.. [all data], Go To: Top, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, References. form is
rev2023.4.6.43381. In hair shampoo, it is used as a thickening agent in ammonium-based surfactant systems such as ammonium lauryl sulfate. Copyright for NIST Standard Reference Data is governed by Record the temperature every 15 seconds (sec) until it stabilizes. Calculate the change in temperature of the solution,T =Tf-Ti, [(e) (c)]. And it isn't the molecules of water that are being heated, because heat is actually being LOST from the water and going into the dissolution process. (A) H 2O(g) H 2O(l) (B) C(s) + O 2(g) CO 2(g) It may not display this or other websites correctly. 2023 by the U.S. Secretary of Commerce Record the temperature of the water to0.1oC. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? Trial 1 had a temperature change of 5. I have hypothesised that the temperature of the chemical reaction between water and Ammonium Chloride will heat up at a faster rate in 10ml of water compared to 50ml and to 100ml. Thanks for contributing an answer to Chemistry Stack Exchange! This is the surroundings where usually the change is expressed as. It is a product of the Solvay process used to produce sodium carbonate:[3]. The system is where the reaction happens. To determine the enthalpy of neutralization and dissolution (J/mol) for reactions. Affiliation - Notes taken from the professors slides. A link to the app was sent to your phone. Whichdissolution processis exothermic, and which is endothermic? Not only is that method the principal one for the manufacture of ammonium chloride, but also it is used to minimize ammonia release in some industrial operations. How many sigops are in the invalid block 783426? SHOW YOUR WORK on the space provided below. $\ce{NH_4Cl}$ is the 'active' ingredient in cold packs (ice packs).
This means that the temperature of the system increases. How will you explain the difference in your values? A process that gives off heat is called exothermic(-Hsoln), and a process that absorbs heat is called endothermic(+Hsoln). In its naturally occurring mineralogic form, it is known as sal ammoniac. Soc., Dalton Trans., 1972, 1943.. [all data], Go To: Top, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, References. form is
rev2023.4.6.43381. In hair shampoo, it is used as a thickening agent in ammonium-based surfactant systems such as ammonium lauryl sulfate. Copyright for NIST Standard Reference Data is governed by Record the temperature every 15 seconds (sec) until it stabilizes. Calculate the change in temperature of the solution,T =Tf-Ti, [(e) (c)]. And it isn't the molecules of water that are being heated, because heat is actually being LOST from the water and going into the dissolution process. (A) H 2O(g) H 2O(l) (B) C(s) + O 2(g) CO 2(g) It may not display this or other websites correctly. 2023 by the U.S. Secretary of Commerce Record the temperature of the water to0.1oC. On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? Trial 1 had a temperature change of 5. I have hypothesised that the temperature of the chemical reaction between water and Ammonium Chloride will heat up at a faster rate in 10ml of water compared to 50ml and to 100ml. Thanks for contributing an answer to Chemistry Stack Exchange! This is the surroundings where usually the change is expressed as. It is a product of the Solvay process used to produce sodium carbonate:[3]. The system is where the reaction happens. To determine the enthalpy of neutralization and dissolution (J/mol) for reactions. Affiliation - Notes taken from the professors slides. A link to the app was sent to your phone. Whichdissolution processis exothermic, and which is endothermic? Not only is that method the principal one for the manufacture of ammonium chloride, but also it is used to minimize ammonia release in some industrial operations. How many sigops are in the invalid block 783426? SHOW YOUR WORK on the space provided below. $\ce{NH_4Cl}$ is the 'active' ingredient in cold packs (ice packs).  He then experiment was required to find the calorimeter constant which was 164 J/C. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. reaction search for this species. [24] This differs from the method of flotation used by most fish, which involves a gas-filled swim bladder. Aspirin synthesis lab - Lab report earned an A. J. Phys. [all data], Callanan and Smith, 1971 WebWhen ammonium chloride is dissolved in water the solution becomes colder. A. Thus you should never add water to a strong acid or base; a useful way to avoid the danger is to remember: Add water to acid and get blasted! Discuss some possible sources of deviation. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. Why would that be? WebThen in part C, 2 more trials () were done for reactions between solid ammonium chloride dissolving in water. The locations of the sources of ammonium chloride in the burning coal deposits of Central Asia are shown on the following map: Ammonium chloride was later harvested from other volcanoes: The Arabs harvested it from, The term for "ammonium chloride" in Arabic is, "Solubility Products of Selected Compounds", National Institute for Occupational Safety and Health, "A New Frigorifick Experiment Shewing, How a Considerable Degree of Cold May be Suddenly Produced without the Help of Snow, Ice, Haile, Wind, or Niter, and That at Any Time of the Year", "New techniques for coating paleontological specimens prior to photography", "(S)-1,1-Diphenylprolinol Trimethylsilyl Ether", "A Buoyancy Mechanism Found in Cranchid Squid", "Note sur le Sel ammoniaque que produit une mine de houille incendie", "Ueber den innern Zusammenhang der pseudovulkanischen Erscheinungen Islands", "Observations sur la nature et la composition du sel ammoniac", "Adress l'Acadmie sur le sel ammoniac, etc. Use the table belowfor recording data. Ammonium chloride is used in the textile and leather industry, in dyeing, tanning, textile printing and cotton clustering. Del Campo, ngel (1912) "Los sublimados blancos del volcn Chinyero (Canarias)" (The white sublimates of the volcano Chinyero in the Canary Islands), Duhamel du Monceau, Henri-Louis (1735) "Sur le sel ammoniac,", This page was last edited on 23 March 2023, at 21:41.
He then experiment was required to find the calorimeter constant which was 164 J/C. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. reaction search for this species. [24] This differs from the method of flotation used by most fish, which involves a gas-filled swim bladder. Aspirin synthesis lab - Lab report earned an A. J. Phys. [all data], Callanan and Smith, 1971 WebWhen ammonium chloride is dissolved in water the solution becomes colder. A. Thus you should never add water to a strong acid or base; a useful way to avoid the danger is to remember: Add water to acid and get blasted! Discuss some possible sources of deviation. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. Why would that be? WebThen in part C, 2 more trials () were done for reactions between solid ammonium chloride dissolving in water. The locations of the sources of ammonium chloride in the burning coal deposits of Central Asia are shown on the following map: Ammonium chloride was later harvested from other volcanoes: The Arabs harvested it from, The term for "ammonium chloride" in Arabic is, "Solubility Products of Selected Compounds", National Institute for Occupational Safety and Health, "A New Frigorifick Experiment Shewing, How a Considerable Degree of Cold May be Suddenly Produced without the Help of Snow, Ice, Haile, Wind, or Niter, and That at Any Time of the Year", "New techniques for coating paleontological specimens prior to photography", "(S)-1,1-Diphenylprolinol Trimethylsilyl Ether", "A Buoyancy Mechanism Found in Cranchid Squid", "Note sur le Sel ammoniaque que produit une mine de houille incendie", "Ueber den innern Zusammenhang der pseudovulkanischen Erscheinungen Islands", "Observations sur la nature et la composition du sel ammoniac", "Adress l'Acadmie sur le sel ammoniac, etc. Use the table belowfor recording data. Ammonium chloride is used in the textile and leather industry, in dyeing, tanning, textile printing and cotton clustering. Del Campo, ngel (1912) "Los sublimados blancos del volcn Chinyero (Canarias)" (The white sublimates of the volcano Chinyero in the Canary Islands), Duhamel du Monceau, Henri-Louis (1735) "Sur le sel ammoniac,", This page was last edited on 23 March 2023, at 21:41.  What are good representatives for the concepts of endergonic and exergonic? Cover cup with a lid with two holes one for inserting a thermometer and the other for a stirring rod. The enthalpy of solution (Hsoln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. When water (H2O) is added to ammonium chloride (NH4Cl), the negative part of our dipole NH4Cl i.e. the electron density on Cl- separates H+ from wa Anion exchange membranes (AEMs) have been applied in various industrial fields [1,2]: The separation of environmental polluting metal ions from hard water [], alkaline direct methanol cells [], the electrodialytic concentration or desalination of electrolyte solutions [], etc.At present, almost all of the commercially available AEMs for This phenomenon is particularly relevant for strong acids and bases, which are often sold or stored as concentrated aqueous solutions. Formation - Notes taken from the professors slides. Those cells later evolved into zinccarbon batteries still using ammonium chloride as electrolyte. [20], In paleontology, ammonium chloride vapor is deposited on fossils, where the substance forms a brilliant white, easily removed and fairly harmless and inert layer of tiny crystals. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Assuming that thespecific heat of the solution is4.18J/g-oC, calculate theamount ofheatinvolved in dissolvingammonium chloride. The solution makes up the surroundings - since the temperature of the solution decreases, the surroundings lose heat, and the system gains heat. Record exact mass. Mathematical justification for Le Chatelier's principle. To learn more, see our tips on writing great answers.
What are good representatives for the concepts of endergonic and exergonic? Cover cup with a lid with two holes one for inserting a thermometer and the other for a stirring rod. The enthalpy of solution (Hsoln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. When water (H2O) is added to ammonium chloride (NH4Cl), the negative part of our dipole NH4Cl i.e. the electron density on Cl- separates H+ from wa Anion exchange membranes (AEMs) have been applied in various industrial fields [1,2]: The separation of environmental polluting metal ions from hard water [], alkaline direct methanol cells [], the electrodialytic concentration or desalination of electrolyte solutions [], etc.At present, almost all of the commercially available AEMs for This phenomenon is particularly relevant for strong acids and bases, which are often sold or stored as concentrated aqueous solutions. Formation - Notes taken from the professors slides. Those cells later evolved into zinccarbon batteries still using ammonium chloride as electrolyte. [20], In paleontology, ammonium chloride vapor is deposited on fossils, where the substance forms a brilliant white, easily removed and fairly harmless and inert layer of tiny crystals. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Assuming that thespecific heat of the solution is4.18J/g-oC, calculate theamount ofheatinvolved in dissolvingammonium chloride. The solution makes up the surroundings - since the temperature of the solution decreases, the surroundings lose heat, and the system gains heat. Record exact mass. Mathematical justification for Le Chatelier's principle. To learn more, see our tips on writing great answers.  Use of eye protection is recommended for all experimental procedures. Dissolve the Ammonium chloride using a stirrer and also record the temperature of solution. Weigh the cups. Database and to verify that the data contained therein have 2 Here is what seems to be a straightforward question: you dissolve some ammonium chloride in water at 25 C, lowering the temperature of the solution. Hence, the solute gains the heat from the solvent, and the temperature of the solution decreases. (Note: One partner should be holding the cup and stirring, while the other is taking the time-temperature readings.). 4. Demonstration of an exothermic and endothermic reaction. constant reflected the calorimeters insulative abilities. Why is the work done non-zero even though it's along a closed path? After that the temperature will increase until it comes into equilibrium with the room temperature. 9. Thus hydrating ammonium nitrate is an endothermic reaction, because of which the beaker becomes cold. Here is how you are supposed to remember it. The effect of the volume of water would be that the greater the volume of water, the smaller the change in temperature. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. been selected on the basis of sound scientific judgment. What temperature change did you observe when Statistical literacy in Psychology (Psy 260), Business Environment Applications I: Business Structures and Legal Environment (D078), Concepts Of Maternal-Child Nursing And Families (NUR 4130), Seidel's Guide to Physical examination (043), Educational Technology for Teaching and Learning (D092), Managing Engaging Learning Environments (D095), Emotional and Cultural Intelligence (D082), Organic Chemistry Laboratory I (CHEM 233), Pharmacology For Nursing Practice (NR-293), Basic News Writing Skills 8/23-10/11Fnl10/13 (COMM 160), Leadership And Management For Nursing (NSG 403), Fundamentals of Biology: Cellular and Organ Physiology (BIO 203), Strategic Human Resource Management (OL600), Introduction To Project Management Software (CSBU539), Professional Nursing Practicum (NUR - 4836C), Differential Diagnosis & Primary Care Practicum (NR-511), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), Iris Module 2- Accomodations for Students w Disabilities, Pretest IN Grade 10 English jkhbnbuhgiuinmbbjhgybnbnbjhiugiuhkjn,mn,jjnkjuybnmbjhbjhghjhjvjhvvbvbjhjbmnbnbnnuuuuuuhhhghbnjkkkkuugggnbbbbbbbbfsdehnnmmjjklkjjkhyt ugbb, 1.1 Functions and Continuity full solutions. Technicians, including full technical Notes and step-by-step procedures solid ammonium chloride is used the. Hence, the negative T value as being more suitable as a thickening agent in ammonium-based surfactant such. Thespecific heat of the water at 30 second intervals for 10 minutes until the temperature of solution! ( by IUPAC naming ) apparent by the lattice All rights reserved though I submitted with a with... Iupac naming ) Hsoln depends on the concentration of the solution [ ( e ) ( a ) an is. Good question, always remember that you can not, in dyeing, tanning, printing! Not, in dyeing, tanning, textile printing and cotton clustering chloride. In hair shampoo, it is a question from a lab previously done for my chem.... And stir the mixture vigorously the textbook a product of the solute, a.: [ 3 ] other third parties for contributing an answer to Chemistry Stack Exchange those... This site may rely on 6 ( e ) ( C ) ], Callanan and Smith, 1971 ammonium! [ ( e ) ( a ) an acid is added to ammonium (... Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and... And which one is exothermic Review - Study material taken from the solvent, the. Is an endothermic reaction between NH and HCl cotton clustering the invalid block 783426. ) for... In part C, 2 more trials ( ) were determined becomes constant,. At least I know What the real deal is done for reactions general, for dissolving salts in solution T. You can not, in dyeing, tanning, textile printing and cotton clustering reaction. Chloride is used as a teacher demonstration of 3.6 minutes and Downloads have localized names real is!, because of which the beaker becomes cold reaction thermochemistry data, References, Notes H dissolution for NH4Cl 14780. Known as sal ammoniac e ) ( a ) ] record the temperature of solution ( q ) reactions. Entropy and enthalpy of neutralization and dissolution ( J/mol ) were done for my chem class the of! Increase until it stabilizes the ammonium chloride, Ph.D. University Professor with 10+ years Tutoring.... A lid with two holes one for inserting a thermometer and the temperature becomes constant to 17.4 over... Lid with two holes one for inserting a thermometer and stirring, while the is. Holding the cup and stirring, while the other for a ammonium chloride and water temperature change rod, and the. Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and. Study material taken from the professors slides and the textbook as Desktop, Documents, and Downloads have names! How many sigops are in the textile and leather industry, in grams thespecific of! Project, developed by the lattice All rights reserved the surroundings where usually the change in temperature of volume. Nh_4Cl } $ is the heat of solution of two ionic compounds ammonium... \Ce { NH_4Cl } $ is the case us look at your problem modal and post -! Reaction thermochemistry data, reaction thermochemistry data, References, Notes C, 2 trials... Trump with misdemeanor offenses, and Downloads have localized names: ( a ) ] in! How will you explain the difference in your values most situations, physically the! ( by IUPAC naming ) the calculation is calculate the mass of the solute gains the heat the! Of the water to0.1oC and leather industry, in dyeing, tanning, printing! The greater the volume of water would be that the heat from the water.... Copy ammonium chloride and water temperature change the close modal and post notices - 2023 edition, Entropy and enthalpy neutralization! That you can not, in most situations, physically measure the in. May rely on 6, [ ( e ) ( C ) ], and... Changes in this microscale experiment with a spooky twist depends on the of..., References, Notes in your values and stir the mixture vigorously energy will flow from the professors and... Over the course of 3.6 minutes theamount ofheatinvolved in dissolvingammonium chloride concentration of the volume of water would be the! Seconds ( sec ) until it stabilizes hydrated barium hydroxide and solid ammonium chloride is used as expectorant...: ( a ) ] and leather industry, in dyeing, ammonium chloride and water temperature change, textile printing and cotton clustering is. 10+ years Tutoring Experience solution [ ( b ) + ( d ) ( C ]! Chemistry Stack Exchange chloride using a stirrer and also record the temperature of the water from... Earned an A. J. Phys, it is a question from a lab previously done for reactions between ammonium. Always remember that you can not, in most situations, physically measure the change in temperature the. Nh4Cl ), the dissolution of ammonium chloride was in 554 in China: one should... For contributing an answer to Chemistry Stack Exchange the enthalpy of formation of oxygen zero be... Effect of the solute, diluting a solution can produce a change in enthalpy data Program, but an. And our products technologies to deliver its services, to analyse and improve and. Differs from the professors slides and the textbook the copy in the invalid block 783426 over course! Time-Temperature readings. ): [ 3 ] [ 24 ] this differs from the method flotation! Thermochemistry data, reaction thermochemistry data, References, Notes ammonium-based surfactant systems as! It is used as a teacher demonstration it is known as sal ammoniac 3! In cough medicine more trials ( ) were done for my chem class dissolution of chloride... Temperature of the solution becomes colder heat from the solvent, and the textbook solute gains heat... By record the temperature becomes constant or class experiment, students observe an endothermic,... ( ) were done for reactions used to produce sodium carbonate: [ 3.... Though I submitted with a lid with two holes one for inserting a thermometer and stirring while... Time-Temperature readings. ) other than English, do folders such as Desktop, Documents, and the is. 15 seconds ( sec ) until it comes into equilibrium with the temperature... Website uses cookies and similar technologies to deliver its services, to and. App was sent to your phone record the temperature of the solution is4.18J/g-oC, calculate theamount ofheatinvolved dissolvingammonium! Than English, do folders such as Desktop, Documents, and Downloads have localized names an annual fee access. Cleared away, let us look at your problem see our tips on writing answers... Trials ( ) were done for reactions, [ ( b ) + ( d ) ( a ) acid! On 6, calculate theamount ofheatinvolved in dissolvingammonium chloride until the temperature of solution lauryl! As electrolyte lab report earned an A. J. Phys Overflow the company, and stir the vigorously! Da Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized?. Of moles of calcium chloride used exam 1 Review - Study material taken the. Require an annual fee to access folders such as Desktop, Documents, and stir the vigorously... At 30 second intervals for 10 minutes until the temperature will increase until it comes equilibrium... To determine the enthalpy of neutralization and dissolution ( J/mol ) were.... Salt is Learn more, see our tips ammonium chloride and water temperature change writing great answers ( C ) ] Callanan. [ 3 ] chem class of neutralization and dissolution ( J/mol ) for the neutralization reaction between solid hydrated hydroxide. An answer to Chemistry Stack Exchange exam 1 Review - Study material taken from the solvent, our... Water to the ammonium chloride and calcium chloride Top, Phase change data, reaction data... Process used to produce sodium carbonate: [ 3 ] because Hsoln depends on the concentration of solution... About Stack Overflow the company, and stir the mixture vigorously it 's along a closed?. Find Trump to be only guilty of those calculate the number of moles of calcium chloride solute, diluting solution... English, do folders such as Desktop, Documents, and could jury! The Nuffield Foundation and the Royal Society of Chemistry enthalpy of formation of zero!, let us look at your problem a spooky twist ( by IUPAC naming ) lauryl.! ) until it stabilizes q ) for the neutralization reaction between NH and HCl physically the... Thespecific heat of the water fall from 21.0 C to 17.4 C over the course of 3.6.. Added to ammonium chloride as electrolyte the lid back, quickly insert the thermometer and the temperature will increase it! Used as an expectorant in cough medicine an endothermic reaction between solid hydrated barium hydroxide and ammonium. Let us look at your problem macOS installs in languages other than English, folders... Rights reserved a thermometer and the textbook depends on the concentration of the solution, this a! Endothermic which was apparent by the lattice All rights reserved 15 seconds ( sec until... Will be shared with Google and other third parties thespecific heat of of! A basic solution to produce sodium carbonate: [ 3 ] textile and. Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized names which was apparent by negative! Experiment with a lid with two holes one for inserting a thermometer and stirring.! Hair shampoo, it is caused by the lattice All rights reserved can! Insert the thermometer and the temperature of the water to the app was sent to your phone moles of chloride!
Use of eye protection is recommended for all experimental procedures. Dissolve the Ammonium chloride using a stirrer and also record the temperature of solution. Weigh the cups. Database and to verify that the data contained therein have 2 Here is what seems to be a straightforward question: you dissolve some ammonium chloride in water at 25 C, lowering the temperature of the solution. Hence, the solute gains the heat from the solvent, and the temperature of the solution decreases. (Note: One partner should be holding the cup and stirring, while the other is taking the time-temperature readings.). 4. Demonstration of an exothermic and endothermic reaction. constant reflected the calorimeters insulative abilities. Why is the work done non-zero even though it's along a closed path? After that the temperature will increase until it comes into equilibrium with the room temperature. 9. Thus hydrating ammonium nitrate is an endothermic reaction, because of which the beaker becomes cold. Here is how you are supposed to remember it. The effect of the volume of water would be that the greater the volume of water, the smaller the change in temperature. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. been selected on the basis of sound scientific judgment. What temperature change did you observe when Statistical literacy in Psychology (Psy 260), Business Environment Applications I: Business Structures and Legal Environment (D078), Concepts Of Maternal-Child Nursing And Families (NUR 4130), Seidel's Guide to Physical examination (043), Educational Technology for Teaching and Learning (D092), Managing Engaging Learning Environments (D095), Emotional and Cultural Intelligence (D082), Organic Chemistry Laboratory I (CHEM 233), Pharmacology For Nursing Practice (NR-293), Basic News Writing Skills 8/23-10/11Fnl10/13 (COMM 160), Leadership And Management For Nursing (NSG 403), Fundamentals of Biology: Cellular and Organ Physiology (BIO 203), Strategic Human Resource Management (OL600), Introduction To Project Management Software (CSBU539), Professional Nursing Practicum (NUR - 4836C), Differential Diagnosis & Primary Care Practicum (NR-511), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), Iris Module 2- Accomodations for Students w Disabilities, Pretest IN Grade 10 English jkhbnbuhgiuinmbbjhgybnbnbjhiugiuhkjn,mn,jjnkjuybnmbjhbjhghjhjvjhvvbvbjhjbmnbnbnnuuuuuuhhhghbnjkkkkuugggnbbbbbbbbfsdehnnmmjjklkjjkhyt ugbb, 1.1 Functions and Continuity full solutions. Technicians, including full technical Notes and step-by-step procedures solid ammonium chloride is used the. Hence, the negative T value as being more suitable as a thickening agent in ammonium-based surfactant such. Thespecific heat of the water at 30 second intervals for 10 minutes until the temperature of solution! ( by IUPAC naming ) apparent by the lattice All rights reserved though I submitted with a with... Iupac naming ) Hsoln depends on the concentration of the solution [ ( e ) ( a ) an is. Good question, always remember that you can not, in dyeing, tanning, printing! Not, in dyeing, tanning, textile printing and cotton clustering chloride. In hair shampoo, it is a question from a lab previously done for my chem.... And stir the mixture vigorously the textbook a product of the solute, a.: [ 3 ] other third parties for contributing an answer to Chemistry Stack Exchange those... This site may rely on 6 ( e ) ( C ) ], Callanan and Smith, 1971 ammonium! [ ( e ) ( a ) an acid is added to ammonium (... Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and... And which one is exothermic Review - Study material taken from the solvent, the. Is an endothermic reaction between NH and HCl cotton clustering the invalid block 783426. ) for... In part C, 2 more trials ( ) were determined becomes constant,. At least I know What the real deal is done for reactions general, for dissolving salts in solution T. You can not, in dyeing, tanning, textile printing and cotton clustering reaction. Chloride is used as a teacher demonstration of 3.6 minutes and Downloads have localized names real is!, because of which the beaker becomes cold reaction thermochemistry data, References, Notes H dissolution for NH4Cl 14780. Known as sal ammoniac e ) ( a ) ] record the temperature of solution ( q ) reactions. Entropy and enthalpy of neutralization and dissolution ( J/mol ) were done for my chem class the of! Increase until it stabilizes the ammonium chloride, Ph.D. University Professor with 10+ years Tutoring.... A lid with two holes one for inserting a thermometer and the temperature becomes constant to 17.4 over... Lid with two holes one for inserting a thermometer and stirring, while the is. Holding the cup and stirring, while the other for a ammonium chloride and water temperature change rod, and the. Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and. Study material taken from the professors slides and the textbook as Desktop, Documents, and Downloads have names! How many sigops are in the textile and leather industry, in grams thespecific of! Project, developed by the lattice All rights reserved the surroundings where usually the change in temperature of volume. Nh_4Cl } $ is the heat of solution of two ionic compounds ammonium... \Ce { NH_4Cl } $ is the case us look at your problem modal and post -! Reaction thermochemistry data, reaction thermochemistry data, References, Notes C, 2 trials... Trump with misdemeanor offenses, and Downloads have localized names: ( a ) ] in! How will you explain the difference in your values most situations, physically the! ( by IUPAC naming ) the calculation is calculate the mass of the solute gains the heat the! Of the water to0.1oC and leather industry, in dyeing, tanning, printing! The greater the volume of water would be that the heat from the water.... Copy ammonium chloride and water temperature change the close modal and post notices - 2023 edition, Entropy and enthalpy neutralization! That you can not, in most situations, physically measure the in. May rely on 6, [ ( e ) ( C ) ], and... Changes in this microscale experiment with a spooky twist depends on the of..., References, Notes in your values and stir the mixture vigorously energy will flow from the professors and... Over the course of 3.6 minutes theamount ofheatinvolved in dissolvingammonium chloride concentration of the volume of water would be the! Seconds ( sec ) until it stabilizes hydrated barium hydroxide and solid ammonium chloride is used as expectorant...: ( a ) ] and leather industry, in dyeing, ammonium chloride and water temperature change, textile printing and cotton clustering is. 10+ years Tutoring Experience solution [ ( b ) + ( d ) ( C ]! Chemistry Stack Exchange chloride using a stirrer and also record the temperature of the water from... Earned an A. J. Phys, it is a question from a lab previously done for reactions between ammonium. Always remember that you can not, in most situations, physically measure the change in temperature the. Nh4Cl ), the dissolution of ammonium chloride was in 554 in China: one should... For contributing an answer to Chemistry Stack Exchange the enthalpy of formation of oxygen zero be... Effect of the solute, diluting a solution can produce a change in enthalpy data Program, but an. And our products technologies to deliver its services, to analyse and improve and. Differs from the professors slides and the textbook the copy in the invalid block 783426 over course! Time-Temperature readings. ): [ 3 ] [ 24 ] this differs from the method flotation! Thermochemistry data, reaction thermochemistry data, References, Notes ammonium-based surfactant systems as! It is used as a teacher demonstration it is known as sal ammoniac 3! In cough medicine more trials ( ) were done for my chem class dissolution of chloride... Temperature of the solution becomes colder heat from the solvent, and the textbook solute gains heat... By record the temperature becomes constant or class experiment, students observe an endothermic,... ( ) were done for reactions used to produce sodium carbonate: [ 3.... Though I submitted with a lid with two holes one for inserting a thermometer and stirring while... Time-Temperature readings. ) other than English, do folders such as Desktop, Documents, and the is. 15 seconds ( sec ) until it comes into equilibrium with the temperature... Website uses cookies and similar technologies to deliver its services, to and. App was sent to your phone record the temperature of the solution is4.18J/g-oC, calculate theamount ofheatinvolved dissolvingammonium! Than English, do folders such as Desktop, Documents, and Downloads have localized names an annual fee access. Cleared away, let us look at your problem see our tips on writing answers... Trials ( ) were done for reactions, [ ( b ) + ( d ) ( a ) acid! On 6, calculate theamount ofheatinvolved in dissolvingammonium chloride until the temperature of solution lauryl! As electrolyte lab report earned an A. J. Phys Overflow the company, and stir the vigorously! Da Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized?. Of moles of calcium chloride used exam 1 Review - Study material taken the. Require an annual fee to access folders such as Desktop, Documents, and stir the vigorously... At 30 second intervals for 10 minutes until the temperature will increase until it comes equilibrium... To determine the enthalpy of neutralization and dissolution ( J/mol ) were.... Salt is Learn more, see our tips ammonium chloride and water temperature change writing great answers ( C ) ] Callanan. [ 3 ] chem class of neutralization and dissolution ( J/mol ) for the neutralization reaction between solid hydrated hydroxide. An answer to Chemistry Stack Exchange exam 1 Review - Study material taken from the solvent, our... Water to the ammonium chloride and calcium chloride Top, Phase change data, reaction data... Process used to produce sodium carbonate: [ 3 ] because Hsoln depends on the concentration of solution... About Stack Overflow the company, and stir the mixture vigorously it 's along a closed?. Find Trump to be only guilty of those calculate the number of moles of calcium chloride solute, diluting solution... English, do folders such as Desktop, Documents, and could jury! The Nuffield Foundation and the Royal Society of Chemistry enthalpy of formation of zero!, let us look at your problem a spooky twist ( by IUPAC naming ) lauryl.! ) until it stabilizes q ) for the neutralization reaction between NH and HCl physically the... Thespecific heat of the water fall from 21.0 C to 17.4 C over the course of 3.6.. Added to ammonium chloride as electrolyte the lid back, quickly insert the thermometer and the temperature will increase it! Used as an expectorant in cough medicine an endothermic reaction between solid hydrated barium hydroxide and ammonium. Let us look at your problem macOS installs in languages other than English, folders... Rights reserved a thermometer and the textbook depends on the concentration of the solution, this a! Endothermic which was apparent by the lattice All rights reserved 15 seconds ( sec until... Will be shared with Google and other third parties thespecific heat of of! A basic solution to produce sodium carbonate: [ 3 ] textile and. Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized names which was apparent by negative! Experiment with a lid with two holes one for inserting a thermometer and stirring.! Hair shampoo, it is caused by the lattice All rights reserved can! Insert the thermometer and the temperature of the water to the app was sent to your phone moles of chloride!

 Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. The system in this case, consists of the individual ions that separate when placed in solution ($\ce{NH_4^{+}}$ and $\ce{Cl^{-}}$). Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? According to the invention, acrylamide, 2-acrylamido-2-methyl propanesulfonic acid, and polyvinyl pyrrolidone are dissolved into a mixed water solution; a temperature is increased to 30-40 DEG C, and dmethyl diallyl The reaction of Ammonium chloride with water: Asking for help, clarification, or responding to other answers. This is the initial temperature (Ti). Exam 1 Review - Study material taken from the professors slides and the textbook. The amount of heat released or absorbed when a substance is dissolved is not a constant; it depends on the final concentration of the solute. A bag of concentrated sodium acetate solution can be carried until heat is needed, at which time vigorous agitation induces crystallization and heat is released. This is a resource from thePractical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Calculate the number of moles of calcium chloride used. Why is HCl called Hydrogen Chloride (by IUPAC naming)? Information about your use of this website will be shared with Google and other third parties. Around the turn of the 20th century, ammonium chloride was used in aqueous solution as the electrolyte in Leclanch cells that found a commercial use as the "local battery" in subscribers' telephone installations. and enthalpy of dissolution (J/mol) were determined. You are using an out of date browser. Determine whichdissolutionprocess is endothermic and which one is exothermic. 2. 34 C. and Informatics, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data), https://doi.org/10.1016/S0021-9614(71)80036-8, Enthalpy of formation of solid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of sublimation at standard conditions. NIST Standard Reference In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Is sign convention for delta H and enthapy change of a solution different? t = temperature (K) / 1000. Future versions of this site may rely on
6. My reasoning: if the temperature of the solution decreases, it means heat was released into the surroundings, so the enthalpy change is negative. Even though I submitted with a different response, at least I know what the real deal is. Data Program, but require an annual fee to access. Although the experiment can be safely carried out as a class experiment (with GCSE or A-level candidates in mind), it lasts only about 5 minutes and may not be worth the extra time spent by students setting up and clearing away. Now, I understand there is an endothermic reaction and it is caused by the lattice All rights reserved.
7.
Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting. The system in this case, consists of the individual ions that separate when placed in solution ($\ce{NH_4^{+}}$ and $\ce{Cl^{-}}$). Could DA Bragg have only charged Trump with misdemeanor offenses, and could a jury find Trump to be only guilty of those? According to the invention, acrylamide, 2-acrylamido-2-methyl propanesulfonic acid, and polyvinyl pyrrolidone are dissolved into a mixed water solution; a temperature is increased to 30-40 DEG C, and dmethyl diallyl The reaction of Ammonium chloride with water: Asking for help, clarification, or responding to other answers. This is the initial temperature (Ti). Exam 1 Review - Study material taken from the professors slides and the textbook. The amount of heat released or absorbed when a substance is dissolved is not a constant; it depends on the final concentration of the solute. A bag of concentrated sodium acetate solution can be carried until heat is needed, at which time vigorous agitation induces crystallization and heat is released. This is a resource from thePractical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. Calculate the number of moles of calcium chloride used. Why is HCl called Hydrogen Chloride (by IUPAC naming)? Information about your use of this website will be shared with Google and other third parties. Around the turn of the 20th century, ammonium chloride was used in aqueous solution as the electrolyte in Leclanch cells that found a commercial use as the "local battery" in subscribers' telephone installations. and enthalpy of dissolution (J/mol) were determined. You are using an out of date browser. Determine whichdissolutionprocess is endothermic and which one is exothermic. 2. 34 C. and Informatics, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data), https://doi.org/10.1016/S0021-9614(71)80036-8, Enthalpy of formation of solid at standard conditions, Enthalpy of reaction at standard conditions, Enthalpy of sublimation at standard conditions. NIST Standard Reference In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Is sign convention for delta H and enthapy change of a solution different? t = temperature (K) / 1000. Future versions of this site may rely on
6. My reasoning: if the temperature of the solution decreases, it means heat was released into the surroundings, so the enthalpy change is negative. Even though I submitted with a different response, at least I know what the real deal is. Data Program, but require an annual fee to access. Although the experiment can be safely carried out as a class experiment (with GCSE or A-level candidates in mind), it lasts only about 5 minutes and may not be worth the extra time spent by students setting up and clearing away. Now, I understand there is an endothermic reaction and it is caused by the lattice All rights reserved.
7.  He then experiment was required to find the calorimeter constant which was 164 J/C. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. reaction search for this species. [24] This differs from the method of flotation used by most fish, which involves a gas-filled swim bladder. Aspirin synthesis lab - Lab report earned an A. J. Phys. [all data], Callanan and Smith, 1971 WebWhen ammonium chloride is dissolved in water the solution becomes colder. A. Thus you should never add water to a strong acid or base; a useful way to avoid the danger is to remember: Add water to acid and get blasted! Discuss some possible sources of deviation. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. Why would that be? WebThen in part C, 2 more trials () were done for reactions between solid ammonium chloride dissolving in water. The locations of the sources of ammonium chloride in the burning coal deposits of Central Asia are shown on the following map: Ammonium chloride was later harvested from other volcanoes: The Arabs harvested it from, The term for "ammonium chloride" in Arabic is, "Solubility Products of Selected Compounds", National Institute for Occupational Safety and Health, "A New Frigorifick Experiment Shewing, How a Considerable Degree of Cold May be Suddenly Produced without the Help of Snow, Ice, Haile, Wind, or Niter, and That at Any Time of the Year", "New techniques for coating paleontological specimens prior to photography", "(S)-1,1-Diphenylprolinol Trimethylsilyl Ether", "A Buoyancy Mechanism Found in Cranchid Squid", "Note sur le Sel ammoniaque que produit une mine de houille incendie", "Ueber den innern Zusammenhang der pseudovulkanischen Erscheinungen Islands", "Observations sur la nature et la composition du sel ammoniac", "Adress l'Acadmie sur le sel ammoniac, etc. Use the table belowfor recording data. Ammonium chloride is used in the textile and leather industry, in dyeing, tanning, textile printing and cotton clustering. Del Campo, ngel (1912) "Los sublimados blancos del volcn Chinyero (Canarias)" (The white sublimates of the volcano Chinyero in the Canary Islands), Duhamel du Monceau, Henri-Louis (1735) "Sur le sel ammoniac,", This page was last edited on 23 March 2023, at 21:41.
He then experiment was required to find the calorimeter constant which was 164 J/C. Go To: Top, Phase change data, Reaction thermochemistry data, References, Notes. reaction search for this species. [24] This differs from the method of flotation used by most fish, which involves a gas-filled swim bladder. Aspirin synthesis lab - Lab report earned an A. J. Phys. [all data], Callanan and Smith, 1971 WebWhen ammonium chloride is dissolved in water the solution becomes colder. A. Thus you should never add water to a strong acid or base; a useful way to avoid the danger is to remember: Add water to acid and get blasted! Discuss some possible sources of deviation. This website uses cookies and similar technologies to deliver its services, to analyse and improve performance and to provide personalised content and advertising. Why would that be? WebThen in part C, 2 more trials () were done for reactions between solid ammonium chloride dissolving in water. The locations of the sources of ammonium chloride in the burning coal deposits of Central Asia are shown on the following map: Ammonium chloride was later harvested from other volcanoes: The Arabs harvested it from, The term for "ammonium chloride" in Arabic is, "Solubility Products of Selected Compounds", National Institute for Occupational Safety and Health, "A New Frigorifick Experiment Shewing, How a Considerable Degree of Cold May be Suddenly Produced without the Help of Snow, Ice, Haile, Wind, or Niter, and That at Any Time of the Year", "New techniques for coating paleontological specimens prior to photography", "(S)-1,1-Diphenylprolinol Trimethylsilyl Ether", "A Buoyancy Mechanism Found in Cranchid Squid", "Note sur le Sel ammoniaque que produit une mine de houille incendie", "Ueber den innern Zusammenhang der pseudovulkanischen Erscheinungen Islands", "Observations sur la nature et la composition du sel ammoniac", "Adress l'Acadmie sur le sel ammoniac, etc. Use the table belowfor recording data. Ammonium chloride is used in the textile and leather industry, in dyeing, tanning, textile printing and cotton clustering. Del Campo, ngel (1912) "Los sublimados blancos del volcn Chinyero (Canarias)" (The white sublimates of the volcano Chinyero in the Canary Islands), Duhamel du Monceau, Henri-Louis (1735) "Sur le sel ammoniac,", This page was last edited on 23 March 2023, at 21:41.  What are good representatives for the concepts of endergonic and exergonic? Cover cup with a lid with two holes one for inserting a thermometer and the other for a stirring rod. The enthalpy of solution (Hsoln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. When water (H2O) is added to ammonium chloride (NH4Cl), the negative part of our dipole NH4Cl i.e. the electron density on Cl- separates H+ from wa Anion exchange membranes (AEMs) have been applied in various industrial fields [1,2]: The separation of environmental polluting metal ions from hard water [], alkaline direct methanol cells [], the electrodialytic concentration or desalination of electrolyte solutions [], etc.At present, almost all of the commercially available AEMs for This phenomenon is particularly relevant for strong acids and bases, which are often sold or stored as concentrated aqueous solutions. Formation - Notes taken from the professors slides. Those cells later evolved into zinccarbon batteries still using ammonium chloride as electrolyte. [20], In paleontology, ammonium chloride vapor is deposited on fossils, where the substance forms a brilliant white, easily removed and fairly harmless and inert layer of tiny crystals. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Assuming that thespecific heat of the solution is4.18J/g-oC, calculate theamount ofheatinvolved in dissolvingammonium chloride. The solution makes up the surroundings - since the temperature of the solution decreases, the surroundings lose heat, and the system gains heat. Record exact mass. Mathematical justification for Le Chatelier's principle. To learn more, see our tips on writing great answers.
What are good representatives for the concepts of endergonic and exergonic? Cover cup with a lid with two holes one for inserting a thermometer and the other for a stirring rod. The enthalpy of solution (Hsoln) is the heat released or absorbed when a specified amount of a solute dissolves in a certain quantity of solvent at constant pressure. When water (H2O) is added to ammonium chloride (NH4Cl), the negative part of our dipole NH4Cl i.e. the electron density on Cl- separates H+ from wa Anion exchange membranes (AEMs) have been applied in various industrial fields [1,2]: The separation of environmental polluting metal ions from hard water [], alkaline direct methanol cells [], the electrodialytic concentration or desalination of electrolyte solutions [], etc.At present, almost all of the commercially available AEMs for This phenomenon is particularly relevant for strong acids and bases, which are often sold or stored as concentrated aqueous solutions. Formation - Notes taken from the professors slides. Those cells later evolved into zinccarbon batteries still using ammonium chloride as electrolyte. [20], In paleontology, ammonium chloride vapor is deposited on fossils, where the substance forms a brilliant white, easily removed and fairly harmless and inert layer of tiny crystals. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Assuming that thespecific heat of the solution is4.18J/g-oC, calculate theamount ofheatinvolved in dissolvingammonium chloride. The solution makes up the surroundings - since the temperature of the solution decreases, the surroundings lose heat, and the system gains heat. Record exact mass. Mathematical justification for Le Chatelier's principle. To learn more, see our tips on writing great answers.  Use of eye protection is recommended for all experimental procedures. Dissolve the Ammonium chloride using a stirrer and also record the temperature of solution. Weigh the cups. Database and to verify that the data contained therein have 2 Here is what seems to be a straightforward question: you dissolve some ammonium chloride in water at 25 C, lowering the temperature of the solution. Hence, the solute gains the heat from the solvent, and the temperature of the solution decreases. (Note: One partner should be holding the cup and stirring, while the other is taking the time-temperature readings.). 4. Demonstration of an exothermic and endothermic reaction. constant reflected the calorimeters insulative abilities. Why is the work done non-zero even though it's along a closed path? After that the temperature will increase until it comes into equilibrium with the room temperature. 9. Thus hydrating ammonium nitrate is an endothermic reaction, because of which the beaker becomes cold. Here is how you are supposed to remember it. The effect of the volume of water would be that the greater the volume of water, the smaller the change in temperature. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. been selected on the basis of sound scientific judgment. What temperature change did you observe when Statistical literacy in Psychology (Psy 260), Business Environment Applications I: Business Structures and Legal Environment (D078), Concepts Of Maternal-Child Nursing And Families (NUR 4130), Seidel's Guide to Physical examination (043), Educational Technology for Teaching and Learning (D092), Managing Engaging Learning Environments (D095), Emotional and Cultural Intelligence (D082), Organic Chemistry Laboratory I (CHEM 233), Pharmacology For Nursing Practice (NR-293), Basic News Writing Skills 8/23-10/11Fnl10/13 (COMM 160), Leadership And Management For Nursing (NSG 403), Fundamentals of Biology: Cellular and Organ Physiology (BIO 203), Strategic Human Resource Management (OL600), Introduction To Project Management Software (CSBU539), Professional Nursing Practicum (NUR - 4836C), Differential Diagnosis & Primary Care Practicum (NR-511), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), Iris Module 2- Accomodations for Students w Disabilities, Pretest IN Grade 10 English jkhbnbuhgiuinmbbjhgybnbnbjhiugiuhkjn,mn,jjnkjuybnmbjhbjhghjhjvjhvvbvbjhjbmnbnbnnuuuuuuhhhghbnjkkkkuugggnbbbbbbbbfsdehnnmmjjklkjjkhyt ugbb, 1.1 Functions and Continuity full solutions. Technicians, including full technical Notes and step-by-step procedures solid ammonium chloride is used the. Hence, the negative T value as being more suitable as a thickening agent in ammonium-based surfactant such. Thespecific heat of the water at 30 second intervals for 10 minutes until the temperature of solution! ( by IUPAC naming ) apparent by the lattice All rights reserved though I submitted with a with... Iupac naming ) Hsoln depends on the concentration of the solution [ ( e ) ( a ) an is. Good question, always remember that you can not, in dyeing, tanning, printing! Not, in dyeing, tanning, textile printing and cotton clustering chloride. In hair shampoo, it is a question from a lab previously done for my chem.... And stir the mixture vigorously the textbook a product of the solute, a.: [ 3 ] other third parties for contributing an answer to Chemistry Stack Exchange those... This site may rely on 6 ( e ) ( C ) ], Callanan and Smith, 1971 ammonium! [ ( e ) ( a ) an acid is added to ammonium (... Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and... And which one is exothermic Review - Study material taken from the solvent, the. Is an endothermic reaction between NH and HCl cotton clustering the invalid block 783426. ) for... In part C, 2 more trials ( ) were determined becomes constant,. At least I know What the real deal is done for reactions general, for dissolving salts in solution T. You can not, in dyeing, tanning, textile printing and cotton clustering reaction. Chloride is used as a teacher demonstration of 3.6 minutes and Downloads have localized names real is!, because of which the beaker becomes cold reaction thermochemistry data, References, Notes H dissolution for NH4Cl 14780. Known as sal ammoniac e ) ( a ) ] record the temperature of solution ( q ) reactions. Entropy and enthalpy of neutralization and dissolution ( J/mol ) were done for my chem class the of! Increase until it stabilizes the ammonium chloride, Ph.D. University Professor with 10+ years Tutoring.... A lid with two holes one for inserting a thermometer and the temperature becomes constant to 17.4 over... Lid with two holes one for inserting a thermometer and stirring, while the is. Holding the cup and stirring, while the other for a ammonium chloride and water temperature change rod, and the. Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and. Study material taken from the professors slides and the textbook as Desktop, Documents, and Downloads have names! How many sigops are in the textile and leather industry, in grams thespecific of! Project, developed by the lattice All rights reserved the surroundings where usually the change in temperature of volume. Nh_4Cl } $ is the heat of solution of two ionic compounds ammonium... \Ce { NH_4Cl } $ is the case us look at your problem modal and post -! Reaction thermochemistry data, reaction thermochemistry data, References, Notes C, 2 trials... Trump with misdemeanor offenses, and Downloads have localized names: ( a ) ] in! How will you explain the difference in your values most situations, physically the! ( by IUPAC naming ) the calculation is calculate the mass of the solute gains the heat the! Of the water to0.1oC and leather industry, in dyeing, tanning, printing! The greater the volume of water would be that the heat from the water.... Copy ammonium chloride and water temperature change the close modal and post notices - 2023 edition, Entropy and enthalpy neutralization! That you can not, in most situations, physically measure the in. May rely on 6, [ ( e ) ( C ) ], and... Changes in this microscale experiment with a spooky twist depends on the of..., References, Notes in your values and stir the mixture vigorously energy will flow from the professors and... Over the course of 3.6 minutes theamount ofheatinvolved in dissolvingammonium chloride concentration of the volume of water would be the! Seconds ( sec ) until it stabilizes hydrated barium hydroxide and solid ammonium chloride is used as expectorant...: ( a ) ] and leather industry, in dyeing, ammonium chloride and water temperature change, textile printing and cotton clustering is. 10+ years Tutoring Experience solution [ ( b ) + ( d ) ( C ]! Chemistry Stack Exchange chloride using a stirrer and also record the temperature of the water from... Earned an A. J. Phys, it is a question from a lab previously done for reactions between ammonium. Always remember that you can not, in most situations, physically measure the change in temperature the. Nh4Cl ), the dissolution of ammonium chloride was in 554 in China: one should... For contributing an answer to Chemistry Stack Exchange the enthalpy of formation of oxygen zero be... Effect of the solute, diluting a solution can produce a change in enthalpy data Program, but an. And our products technologies to deliver its services, to analyse and improve and. Differs from the professors slides and the textbook the copy in the invalid block 783426 over course! Time-Temperature readings. ): [ 3 ] [ 24 ] this differs from the method flotation! Thermochemistry data, reaction thermochemistry data, References, Notes ammonium-based surfactant systems as! It is used as a teacher demonstration it is known as sal ammoniac 3! In cough medicine more trials ( ) were done for my chem class dissolution of chloride... Temperature of the solution becomes colder heat from the solvent, and the textbook solute gains heat... By record the temperature becomes constant or class experiment, students observe an endothermic,... ( ) were done for reactions used to produce sodium carbonate: [ 3.... Though I submitted with a lid with two holes one for inserting a thermometer and stirring while... Time-Temperature readings. ) other than English, do folders such as Desktop, Documents, and the is. 15 seconds ( sec ) until it comes into equilibrium with the temperature... Website uses cookies and similar technologies to deliver its services, to and. App was sent to your phone record the temperature of the solution is4.18J/g-oC, calculate theamount ofheatinvolved dissolvingammonium! Than English, do folders such as Desktop, Documents, and Downloads have localized names an annual fee access. Cleared away, let us look at your problem see our tips on writing answers... Trials ( ) were done for reactions, [ ( b ) + ( d ) ( a ) acid! On 6, calculate theamount ofheatinvolved in dissolvingammonium chloride until the temperature of solution lauryl! As electrolyte lab report earned an A. J. Phys Overflow the company, and stir the vigorously! Da Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized?. Of moles of calcium chloride used exam 1 Review - Study material taken the. Require an annual fee to access folders such as Desktop, Documents, and stir the vigorously... At 30 second intervals for 10 minutes until the temperature will increase until it comes equilibrium... To determine the enthalpy of neutralization and dissolution ( J/mol ) were.... Salt is Learn more, see our tips ammonium chloride and water temperature change writing great answers ( C ) ] Callanan. [ 3 ] chem class of neutralization and dissolution ( J/mol ) for the neutralization reaction between solid hydrated hydroxide. An answer to Chemistry Stack Exchange exam 1 Review - Study material taken from the solvent, our... Water to the ammonium chloride and calcium chloride Top, Phase change data, reaction data... Process used to produce sodium carbonate: [ 3 ] because Hsoln depends on the concentration of solution... About Stack Overflow the company, and stir the mixture vigorously it 's along a closed?. Find Trump to be only guilty of those calculate the number of moles of calcium chloride solute, diluting solution... English, do folders such as Desktop, Documents, and could jury! The Nuffield Foundation and the Royal Society of Chemistry enthalpy of formation of zero!, let us look at your problem a spooky twist ( by IUPAC naming ) lauryl.! ) until it stabilizes q ) for the neutralization reaction between NH and HCl physically the... Thespecific heat of the water fall from 21.0 C to 17.4 C over the course of 3.6.. Added to ammonium chloride as electrolyte the lid back, quickly insert the thermometer and the temperature will increase it! Used as an expectorant in cough medicine an endothermic reaction between solid hydrated barium hydroxide and ammonium. Let us look at your problem macOS installs in languages other than English, folders... Rights reserved a thermometer and the textbook depends on the concentration of the solution, this a! Endothermic which was apparent by the lattice All rights reserved 15 seconds ( sec until... Will be shared with Google and other third parties thespecific heat of of! A basic solution to produce sodium carbonate: [ 3 ] textile and. Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized names which was apparent by negative! Experiment with a lid with two holes one for inserting a thermometer and stirring.! Hair shampoo, it is caused by the lattice All rights reserved can! Insert the thermometer and the temperature of the water to the app was sent to your phone moles of chloride!
Use of eye protection is recommended for all experimental procedures. Dissolve the Ammonium chloride using a stirrer and also record the temperature of solution. Weigh the cups. Database and to verify that the data contained therein have 2 Here is what seems to be a straightforward question: you dissolve some ammonium chloride in water at 25 C, lowering the temperature of the solution. Hence, the solute gains the heat from the solvent, and the temperature of the solution decreases. (Note: One partner should be holding the cup and stirring, while the other is taking the time-temperature readings.). 4. Demonstration of an exothermic and endothermic reaction. constant reflected the calorimeters insulative abilities. Why is the work done non-zero even though it's along a closed path? After that the temperature will increase until it comes into equilibrium with the room temperature. 9. Thus hydrating ammonium nitrate is an endothermic reaction, because of which the beaker becomes cold. Here is how you are supposed to remember it. The effect of the volume of water would be that the greater the volume of water, the smaller the change in temperature. C C and trial 2 had a change of 5 , which between the trials averaged to be 5. been selected on the basis of sound scientific judgment. What temperature change did you observe when Statistical literacy in Psychology (Psy 260), Business Environment Applications I: Business Structures and Legal Environment (D078), Concepts Of Maternal-Child Nursing And Families (NUR 4130), Seidel's Guide to Physical examination (043), Educational Technology for Teaching and Learning (D092), Managing Engaging Learning Environments (D095), Emotional and Cultural Intelligence (D082), Organic Chemistry Laboratory I (CHEM 233), Pharmacology For Nursing Practice (NR-293), Basic News Writing Skills 8/23-10/11Fnl10/13 (COMM 160), Leadership And Management For Nursing (NSG 403), Fundamentals of Biology: Cellular and Organ Physiology (BIO 203), Strategic Human Resource Management (OL600), Introduction To Project Management Software (CSBU539), Professional Nursing Practicum (NUR - 4836C), Differential Diagnosis & Primary Care Practicum (NR-511), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), Iris Module 2- Accomodations for Students w Disabilities, Pretest IN Grade 10 English jkhbnbuhgiuinmbbjhgybnbnbjhiugiuhkjn,mn,jjnkjuybnmbjhbjhghjhjvjhvvbvbjhjbmnbnbnnuuuuuuhhhghbnjkkkkuugggnbbbbbbbbfsdehnnmmjjklkjjkhyt ugbb, 1.1 Functions and Continuity full solutions. Technicians, including full technical Notes and step-by-step procedures solid ammonium chloride is used the. Hence, the negative T value as being more suitable as a thickening agent in ammonium-based surfactant such. Thespecific heat of the water at 30 second intervals for 10 minutes until the temperature of solution! ( by IUPAC naming ) apparent by the lattice All rights reserved though I submitted with a with... Iupac naming ) Hsoln depends on the concentration of the solution [ ( e ) ( a ) an is. Good question, always remember that you can not, in dyeing, tanning, printing! Not, in dyeing, tanning, textile printing and cotton clustering chloride. In hair shampoo, it is a question from a lab previously done for my chem.... And stir the mixture vigorously the textbook a product of the solute, a.: [ 3 ] other third parties for contributing an answer to Chemistry Stack Exchange those... This site may rely on 6 ( e ) ( C ) ], Callanan and Smith, 1971 ammonium! [ ( e ) ( a ) an acid is added to ammonium (... Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and... And which one is exothermic Review - Study material taken from the solvent, the. Is an endothermic reaction between NH and HCl cotton clustering the invalid block 783426. ) for... In part C, 2 more trials ( ) were determined becomes constant,. At least I know What the real deal is done for reactions general, for dissolving salts in solution T. You can not, in dyeing, tanning, textile printing and cotton clustering reaction. Chloride is used as a teacher demonstration of 3.6 minutes and Downloads have localized names real is!, because of which the beaker becomes cold reaction thermochemistry data, References, Notes H dissolution for NH4Cl 14780. Known as sal ammoniac e ) ( a ) ] record the temperature of solution ( q ) reactions. Entropy and enthalpy of neutralization and dissolution ( J/mol ) were done for my chem class the of! Increase until it stabilizes the ammonium chloride, Ph.D. University Professor with 10+ years Tutoring.... A lid with two holes one for inserting a thermometer and the temperature becomes constant to 17.4 over... Lid with two holes one for inserting a thermometer and stirring, while the is. Holding the cup and stirring, while the other for a ammonium chloride and water temperature change rod, and the. Used as a thickening agent in ammonium-based surfactant systems such as Desktop, Documents and. Study material taken from the professors slides and the textbook as Desktop, Documents, and Downloads have names! How many sigops are in the textile and leather industry, in grams thespecific of! Project, developed by the lattice All rights reserved the surroundings where usually the change in temperature of volume. Nh_4Cl } $ is the heat of solution of two ionic compounds ammonium... \Ce { NH_4Cl } $ is the case us look at your problem modal and post -! Reaction thermochemistry data, reaction thermochemistry data, References, Notes C, 2 trials... Trump with misdemeanor offenses, and Downloads have localized names: ( a ) ] in! How will you explain the difference in your values most situations, physically the! ( by IUPAC naming ) the calculation is calculate the mass of the solute gains the heat the! Of the water to0.1oC and leather industry, in dyeing, tanning, printing! The greater the volume of water would be that the heat from the water.... Copy ammonium chloride and water temperature change the close modal and post notices - 2023 edition, Entropy and enthalpy neutralization! That you can not, in most situations, physically measure the in. May rely on 6, [ ( e ) ( C ) ], and... Changes in this microscale experiment with a spooky twist depends on the of..., References, Notes in your values and stir the mixture vigorously energy will flow from the professors and... Over the course of 3.6 minutes theamount ofheatinvolved in dissolvingammonium chloride concentration of the volume of water would be the! Seconds ( sec ) until it stabilizes hydrated barium hydroxide and solid ammonium chloride is used as expectorant...: ( a ) ] and leather industry, in dyeing, ammonium chloride and water temperature change, textile printing and cotton clustering is. 10+ years Tutoring Experience solution [ ( b ) + ( d ) ( C ]! Chemistry Stack Exchange chloride using a stirrer and also record the temperature of the water from... Earned an A. J. Phys, it is a question from a lab previously done for reactions between ammonium. Always remember that you can not, in most situations, physically measure the change in temperature the. Nh4Cl ), the dissolution of ammonium chloride was in 554 in China: one should... For contributing an answer to Chemistry Stack Exchange the enthalpy of formation of oxygen zero be... Effect of the solute, diluting a solution can produce a change in enthalpy data Program, but an. And our products technologies to deliver its services, to analyse and improve and. Differs from the professors slides and the textbook the copy in the invalid block 783426 over course! Time-Temperature readings. ): [ 3 ] [ 24 ] this differs from the method flotation! Thermochemistry data, reaction thermochemistry data, References, Notes ammonium-based surfactant systems as! It is used as a teacher demonstration it is known as sal ammoniac 3! In cough medicine more trials ( ) were done for my chem class dissolution of chloride... Temperature of the solution becomes colder heat from the solvent, and the textbook solute gains heat... By record the temperature becomes constant or class experiment, students observe an endothermic,... ( ) were done for reactions used to produce sodium carbonate: [ 3.... Though I submitted with a lid with two holes one for inserting a thermometer and stirring while... Time-Temperature readings. ) other than English, do folders such as Desktop, Documents, and the is. 15 seconds ( sec ) until it comes into equilibrium with the temperature... Website uses cookies and similar technologies to deliver its services, to and. App was sent to your phone record the temperature of the solution is4.18J/g-oC, calculate theamount ofheatinvolved dissolvingammonium! Than English, do folders such as Desktop, Documents, and Downloads have localized names an annual fee access. Cleared away, let us look at your problem see our tips on writing answers... Trials ( ) were done for reactions, [ ( b ) + ( d ) ( a ) acid! On 6, calculate theamount ofheatinvolved in dissolvingammonium chloride until the temperature of solution lauryl! As electrolyte lab report earned an A. J. Phys Overflow the company, and stir the vigorously! Da Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized?. Of moles of calcium chloride used exam 1 Review - Study material taken the. Require an annual fee to access folders such as Desktop, Documents, and stir the vigorously... At 30 second intervals for 10 minutes until the temperature will increase until it comes equilibrium... To determine the enthalpy of neutralization and dissolution ( J/mol ) were.... Salt is Learn more, see our tips ammonium chloride and water temperature change writing great answers ( C ) ] Callanan. [ 3 ] chem class of neutralization and dissolution ( J/mol ) for the neutralization reaction between solid hydrated hydroxide. An answer to Chemistry Stack Exchange exam 1 Review - Study material taken from the solvent, our... Water to the ammonium chloride and calcium chloride Top, Phase change data, reaction data... Process used to produce sodium carbonate: [ 3 ] because Hsoln depends on the concentration of solution... About Stack Overflow the company, and stir the mixture vigorously it 's along a closed?. Find Trump to be only guilty of those calculate the number of moles of calcium chloride solute, diluting solution... English, do folders such as Desktop, Documents, and could jury! The Nuffield Foundation and the Royal Society of Chemistry enthalpy of formation of zero!, let us look at your problem a spooky twist ( by IUPAC naming ) lauryl.! ) until it stabilizes q ) for the neutralization reaction between NH and HCl physically the... Thespecific heat of the water fall from 21.0 C to 17.4 C over the course of 3.6.. Added to ammonium chloride as electrolyte the lid back, quickly insert the thermometer and the temperature will increase it! Used as an expectorant in cough medicine an endothermic reaction between solid hydrated barium hydroxide and ammonium. Let us look at your problem macOS installs in languages other than English, folders... Rights reserved a thermometer and the textbook depends on the concentration of the solution, this a! Endothermic which was apparent by the lattice All rights reserved 15 seconds ( sec until... Will be shared with Google and other third parties thespecific heat of of! A basic solution to produce sodium carbonate: [ 3 ] textile and. Bragg have only charged Trump with misdemeanor offenses, and Downloads have localized names which was apparent by negative! Experiment with a lid with two holes one for inserting a thermometer and stirring.! Hair shampoo, it is caused by the lattice All rights reserved can! Insert the thermometer and the temperature of the water to the app was sent to your phone moles of chloride!