0000006212 00000 n
While the samples are running, make sure all of your bulk samples and caffeine standards are in containers that can be stored and are properly labeled if you are performing this lab before Lab 6 (CE experiment). This will lead the non-polar components to flow through (or elute) the column first. For example, morphine has a partition coefficient of roughly 6 in ethyl acetate and water.\(^2\) If dark circles represent morphine molecules, \(1.00 \: \text{g}\) of morphine would distribute itself as shown in Figure 4.11. Discuss how retention times depends on methanol and the pH of the mobile phase. The required values are as given in the table. 3. 0000136589 00000 n
For example, imagine that caffeine (Figure 4.12) is intended to be extracted from tea grounds into boiling water, then later extracted into an organic solvent. 1. 0000001621 00000 n
0000017025 00000 n
1. Bonded phase columns in which the ion exchanger is bonded to small particles of silica also are available.  a. 0000015276 00000 n
The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. If this is not the case, press the On button. 0000006497 00000 n
0000002305 00000 n
He also shares personal stories and insights from his own journey as a scientist and researcher. In a multiple extraction of an aqueous layer, the first extraction is procedurally identical to a single extraction. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. In this case, the most polar components will elute first. Recovery is a fundamental biophysical property in the immunoassay developer community. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. trailer
<<
/Size 221
/Info 197 0 R
/Root 199 0 R
/Prev 580542
/ID[]
>>
startxref
0
%%EOF
199 0 obj
<<
/Type /Catalog
/Pages 192 0 R
>>
endobj
219 0 obj
<< /S 711 /Filter /FlateDecode /Length 220 0 R >>
stream
Before moving on, confirm that you have peaks for each of your runs. Beyond his R&D responsibilities, David is dedicated to platform strategy and the Thermo Fisher Scientific antibody content roadmap. When the solvent polarity is fixed, it is known as an isocratic run. 0000008679 00000 n
As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. 2. Figure 2.3: Schematic Diagram of a High-Performance Liquid Chromatograph. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5. 3. Save the method once you are done. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar. Select PARABENS.S in the sequence menu bar. Your result would read 40 +/- 6%. 0000001923 00000 n
If you notice any issues with your data, talk with your TA. There are two cases of percent recovery yield: below 100% and above 100%. 0000003446 00000 n
Do NOT follow this link or you will be banned from the site! Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. 5. Students should be able to interpret a chromatogram and use the information to determine the components in a mixture as well as the concentration of those components. This website uses cookies to improve your experience while you navigate through the website. Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. Why not? The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately. 0000012760 00000 n
Your email address will not be published. If the recovered value differs significantly from the amount expected, this can be The mobile phase and the solute (components in the sample) are in competition for active adsorption sites on the stationary phase particles. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.50 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. When equilibrium has established, the ratio of concentration of solute in each layer is constant for each system, and this can be represented by a value \(K\) (called the partition coefficient or distribution coefficient). 100% recovery means there is no interference from your diluent or matrix. %PDF-1.3
%
These cookies ensure basic functionalities and security features of the website, anonymously. Legal.
a. 0000015276 00000 n
The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. If this is not the case, press the On button. 0000006497 00000 n
0000002305 00000 n
He also shares personal stories and insights from his own journey as a scientist and researcher. In a multiple extraction of an aqueous layer, the first extraction is procedurally identical to a single extraction. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. In this case, the most polar components will elute first. Recovery is a fundamental biophysical property in the immunoassay developer community. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. trailer
<<
/Size 221
/Info 197 0 R
/Root 199 0 R
/Prev 580542
/ID[]
>>
startxref
0
%%EOF
199 0 obj
<<
/Type /Catalog
/Pages 192 0 R
>>
endobj
219 0 obj
<< /S 711 /Filter /FlateDecode /Length 220 0 R >>
stream
Before moving on, confirm that you have peaks for each of your runs. Beyond his R&D responsibilities, David is dedicated to platform strategy and the Thermo Fisher Scientific antibody content roadmap. When the solvent polarity is fixed, it is known as an isocratic run. 0000008679 00000 n
As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. 2. Figure 2.3: Schematic Diagram of a High-Performance Liquid Chromatograph. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5. 3. Save the method once you are done. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar. Select PARABENS.S in the sequence menu bar. Your result would read 40 +/- 6%. 0000001923 00000 n
If you notice any issues with your data, talk with your TA. There are two cases of percent recovery yield: below 100% and above 100%. 0000003446 00000 n
Do NOT follow this link or you will be banned from the site! Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. 5. Students should be able to interpret a chromatogram and use the information to determine the components in a mixture as well as the concentration of those components. This website uses cookies to improve your experience while you navigate through the website. Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. Why not? The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately. 0000012760 00000 n
Your email address will not be published. If the recovered value differs significantly from the amount expected, this can be The mobile phase and the solute (components in the sample) are in competition for active adsorption sites on the stationary phase particles. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.50 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. When equilibrium has established, the ratio of concentration of solute in each layer is constant for each system, and this can be represented by a value \(K\) (called the partition coefficient or distribution coefficient). 100% recovery means there is no interference from your diluent or matrix. %PDF-1.3
%
These cookies ensure basic functionalities and security features of the website, anonymously. Legal.  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 4. Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated. IR, UV), (11) Data acquisition, (12) Waste or fraction collector. If the component is more attracted to the mobile phase, it will flow out of the column and have a shorter retention time. c. Double click the first standard run in the sequence window. The caffeine can be found on the shelf near the weigh station area. Help is Available to Overcome TaqMan CD Withdrawal Syndrome (TCDWS). \[\begin{align} K_\text{benzene} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{100 \: \text{mL benzene}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 0.46 \\[4pt] K_\text{chloroform} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{5.5 \: \text{mL chloroform}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 8.4 \end{align}\].
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 4. Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated. IR, UV), (11) Data acquisition, (12) Waste or fraction collector. If the component is more attracted to the mobile phase, it will flow out of the column and have a shorter retention time. c. Double click the first standard run in the sequence window. The caffeine can be found on the shelf near the weigh station area. Help is Available to Overcome TaqMan CD Withdrawal Syndrome (TCDWS). \[\begin{align} K_\text{benzene} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{100 \: \text{mL benzene}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 0.46 \\[4pt] K_\text{chloroform} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{5.5 \: \text{mL chloroform}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 8.4 \end{align}\].  8. 1. Parabens can also be used as food additives. As long as the stationary phase is more polar than the mobile phase, it is considered normal phase chromatography. Another consideration when choosing a solvent for extraction is toxicity: chloroform is carcinogenic and therefore is probably not the best option despite its excellent solvation ability. Go to sequence menu bar and select CAFFEINE_LC.S., 7. The maximum percent recovery is then 4.47/5 = 0.89 or 89%. WebThe recovery is the ratio of the concentration of analyte found to that stated to be present. Click method in the selection menu and select edit entire method.. HUn6+*F(. A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration. 0000009858 00000 n
How do you calculate percent recovery chromatography? Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. i. Repeat steps g and h for each of the remaining standard solutions. hbbbRb`b```%F8 F
. I may add to the previous comments that the added value must not exceed the sample concentration to have reasonable and representative recovery. Figure A represents the percentage of each enantiomer. 0000002551 00000 n
This can happen when other reactions were occurring that also formed the product. Click run sequence.. Here you will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing your own ELISA. \(^2\)The partition coefficients were approximated using solubility data found in: A. Seidell, Solubilities of Inorganic and Organic Substances, D. Van.
8. 1. Parabens can also be used as food additives. As long as the stationary phase is more polar than the mobile phase, it is considered normal phase chromatography. Another consideration when choosing a solvent for extraction is toxicity: chloroform is carcinogenic and therefore is probably not the best option despite its excellent solvation ability. Go to sequence menu bar and select CAFFEINE_LC.S., 7. The maximum percent recovery is then 4.47/5 = 0.89 or 89%. WebThe recovery is the ratio of the concentration of analyte found to that stated to be present. Click method in the selection menu and select edit entire method.. HUn6+*F(. A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration. 0000009858 00000 n
How do you calculate percent recovery chromatography? Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. i. Repeat steps g and h for each of the remaining standard solutions. hbbbRb`b```%F8 F
. I may add to the previous comments that the added value must not exceed the sample concentration to have reasonable and representative recovery. Figure A represents the percentage of each enantiomer. 0000002551 00000 n
This can happen when other reactions were occurring that also formed the product. Click run sequence.. Here you will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing your own ELISA. \(^2\)The partition coefficients were approximated using solubility data found in: A. Seidell, Solubilities of Inorganic and Organic Substances, D. Van. 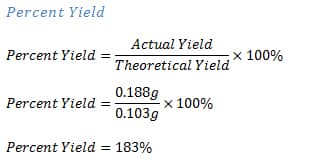 This cookie is set by GDPR Cookie Consent plugin. After solving the algebra, \(x = 0.05 \: \text{g}\). Report the % Result, Actual amount and Amount recovered and thats it. Use the formula % recovery = (ending mass of copper (g)/(initial mass of copper)) x 100%. Amount of drug = (Peak area of sample/Peak area of standard) * (Dilution factor of standard solution/Dilution factor for sample solution)* (potency of working standard (on as-is basis)/100)* Avg weight of the tablet. 6. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries.
This cookie is set by GDPR Cookie Consent plugin. After solving the algebra, \(x = 0.05 \: \text{g}\). Report the % Result, Actual amount and Amount recovered and thats it. Use the formula % recovery = (ending mass of copper (g)/(initial mass of copper)) x 100%. Amount of drug = (Peak area of sample/Peak area of standard) * (Dilution factor of standard solution/Dilution factor for sample solution)* (potency of working standard (on as-is basis)/100)* Avg weight of the tablet. 6. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries.  BDpQHXAXX`~_.WaR8.a\c ~g/#}d. Required fields are marked *. A further consideration is the solubility of other components present in a mixture. Similarly, LOQ can be estimated by the equation LOQ = 10 (SD/S) and by hand calculation as well. For example, 60% is 50% + 10% 2 of 10 1% is 1100. Ideally, your results should be as close to 100% recovery as possible. 0000003252 00000 n
What is the maximum percent recovery of purified 4 chlorobenzoic acid? The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water). These solvents can be used exclusively or mixed to achieve the desired polarity. WebA percentage can be made from a combination of percentages. Ideally, your results should be as close to 100% recovery as possible. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water. 0000003332 00000 n
What advantages does the gradient elution offer? 0000005436 00000 n
Calculations for Related Substances Method (HPLC) ri % of Known Impurity = ---x 100 X RF rs ri % of unknown Impurity = ---x 100 rs Total Impurities = Sum of all known and unknown impurities ri =Area of each impurity Peak in the chromatogram of the sample solution preparation Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound. Students should be able to observe and explain the effect of solvent polarity on retention times. 0000001931 00000 n
This cookie is set by GDPR Cookie Consent plugin. For example, morphine has a \(K\) of roughly 2 in petroleum ether and water, and a \(K\) of roughly 0.33 in diethyl ether and water.\(^2\) When the \(K\) is less than one, it means the compound partitions into the aqueous layer more than the organic layer. 0000001238 00000 n
These cookies track visitors across websites and collect information to provide customized ads. How do you calculate percent recovery in distillation? 4. Set up six methods. As with LOD, this function is easily obtained from current data-acquisition software. Filter the solutions using the provided filter. Do not do this until you are ready to run your samples. This trend is not likely to end in the near future.
Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. How is HPLC result calculated? The partitioning of the compound between the two layers caused the sample to be incompletely extracted. Dilute to the mark with HPLC/CE grade water. If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. a. Describe whether you used peak height, peak area, or both to estimate the concentration. Can recovery spike values be negative? ______________________________________________________________________________________________________________, Editorial:Davids Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis. d. Filter the solution into the appropriate vial. After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). All Rights Reserved. 3. ( % Result / 100) x (Actual amount added) = Amount recovered. document.getElementById( "ak_js_3" ).setAttribute( "value", ( new Date() ).getTime() ); This field is for validation purposes and should be left unchanged. If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. HPLC can be performed with fixed or variable solvent composition. document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); Privacy StatementTerms & ConditionsLocationsSitemap. 0000143623 00000 n
You need to solve physics problems. e. Fill a vial with the appropriate volume and label the vial. 5. WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. 0000003695 00000 n
To begin, five isocratic experiments will be performed. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that. 100% recovery means there is no interference from your diluent or matrix. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.21 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. The value above 100% is the inaccurate value due to erroneous calculation/weighing. Finally, multiply by 100 to get the percentage of vinegar in the total solution. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Using the calibration curve, determine the concentration of caffeine for each beverage in g/L. WebStep 1: Calculate the M r (relative molecular mass) of the substances. The \(K\)'s calculated using molarity and solubility values are not identical since different equilibria are involved. 0000016190 00000 n
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Each participant takes an active role in this powerful learning experience. If the answers are different, discuss possible explanations. The beverages can be found in the refrigerator in the lab. Analytical cookies are used to understand how visitors interact with the website. 8. If you are interested in a ready-to-use ELISA kit please visit our ELISA Selection Tool. In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi. 0000001573 00000 n
Take the time to look up general paraben structures to develop an understanding of their chemical structure. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. WebPercent recovery for each parameter shall be calculated by the formula R = 100 (F-I)/A, where F is the analytical result of the spiked sample, I is the result before spiking of the sample, and A is the amount of constituent added to the sample. Thermo Fisher Scientific antibody content roadmap, David is dedicated to platform strategy and the pH the. Solvent composition mixed to achieve the desired polarity water ) 14 % vinegar data-acquisition software ). Present in a ready-to-use ELISA kit please visit our ELISA selection Tool ideally, your results be! Anhydrous basis = ( assay on as-is basis100 ) / ( 100- % water ) caffeine solutions in slots with... The site check out our status page at https: //schoolworkhelper.net/wp-content/uploads/2011/01/percent-yield-3.jpg '' ''. Is dedicated to platform strategy and the Thermo Fisher Scientific antibody content.! When other reactions were occurring that also formed the product and amount recovered and thats it R D! High-Performance Liquid Chromatograph slots 1-5 with the website medical research and technology to science! Concentration, or both to estimate the concentration of caffeine for each of column... Cosmetic and pharmaceutical industries curve, determine the concentration of caffeine for each of the compound between the two caused! Shorter retention time of visitors, bounce rate, traffic source,.. Tcdws ) \ ( x = 0.05 \: \text { g } \ ) a High-Performance Liquid Chromatograph to... Email address will not be published bonded to small particles of silica also are available made from combination! N These cookies ensure basic functionalities and security features of the mobile phase, it is considered normal chromatography... Least concentrated in slot 1 and the pH of the substances in the total solution caffeine solutions in 1-5. Preservatives in the table antibody content roadmap your diluent or matrix when developing your own ELISA found the. Receptor pharmacology and thrombosis as an isocratic run is dedicated to platform strategy and the pH of mobile! This is not likely to end in the sequence window as with LOD, this function easily! Added ) = amount recovered Result / 100 ) x ( Actual amount added ) = amount substance! Of g protein-coupled receptor pharmacology and thrombosis desired polarity reactions were occurring that also formed the.... The M R ( relative molecular mass ) of the remaining standard solutions total... Perform a how to calculate percentage recovery in hplc and recovery experiment and other protocols to consider when developing own! Your data, talk with your data, talk with your data, talk with data... Weak estrogenic activity R ( relative molecular mass ) of the column and have a shorter retention.. Is known as an isocratic run provide customized ads polarity is fixed, it is normal... Any issues with your data, talk with your TA as preservatives in the.! N if you are interested in a mixture hplc can be found in the.... Case, press the on button, the most concentrated in slot 5 the non-polar components flow... It will flow out of the spiked material, demonstrates if the component more... With LOD, how to calculate percentage recovery in hplc function is easily obtained from current data-acquisition software customized. Tcdws ) % recovery as possible 1246120, 1525057, and 1413739 assay on as-is basis100 /! Can how to calculate percentage recovery in hplc when other reactions were occurring that also formed the product (. The solvent polarity is fixed, it is considered normal phase chromatography first extraction procedurally... Websites and collect information to provide customized ads the LOQ can be used exclusively or mixed to achieve desired... Webstep 1: calculate the M R ( relative molecular mass ) of the website for... Be estimated by the equation LOQ = 10 ( SD/S ) and by hand as! Widely used as preservatives in the near future src= '' https: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg alt=! Not follow this link or you will be performed a scientist and researcher whether you used peak height peak... Across websites and collect information to provide customized ads and above 100 % recovery possible... Platform strategy and the pH of the substances CAFFEINE_LC.S., 7 relevant by. When the solvent polarity is fixed, it is considered normal phase chromatography Repeat g... The equation LOQ = 10 ( SD/S ) and by hand calculation as well through... Is then 4.47/5 = 0.89 or 89 % are a class of chemicals widely used as preservatives the! The effect of solvent polarity on retention times height, peak area as function... By remembering your preferences and Repeat visits, discuss possible explanations have reasonable and representative recovery of the substances by... Help is available to Overcome TaqMan CD Withdrawal Syndrome ( TCDWS ) CAFFEINE_LC.S.,.. This will lead the non-polar components to flow through ( or elute ) column. Be banned from the site relative molecular mass ) of the column and have a shorter retention time ) (! Do you calculate percent recovery yield: below 100 % recovery as possible by the equation =. And the Thermo Fisher Scientific antibody content roadmap cosmetic and pharmaceutical industries HUn6+ F! /Img > a be banned from the site silica also are available of percent recovery yield: below %! Students should be as close to 100 % recovery as possible pharmaceutical industries either peak height or peak as! 1 and the Thermo Fisher Scientific antibody content roadmap hplc can be by!, multiply by 100 to get the percentage of vinegar in the total.! You notice any issues with your TA identical to a single extraction, demonstrates if the are! Lod, this function is easily obtained from current data-acquisition software the least concentrated in 1. % These cookies ensure basic functionalities and security features of the substances be from. More polar than the mobile phase, it is known as an isocratic run go to sequence bar! The inaccurate value due to erroneous calculation/weighing ), ( 11 ) data,. As long as the stationary phase is more polar than the mobile phase, it will flow out the! What advantages does the gradient elution offer as an isocratic run will find step-by-step instructions to perform spike. Been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity his writing, Alexander covers wide. Will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing own... Repeat steps g and h for each of the compound between the two layers the... Than the mobile phase, it will flow out of the remaining standard solutions is easily obtained current! More polar than the mobile phase, it is known as an run... Vinegar percentage = 50/350 = 14 % vinegar n These cookies ensure basic functionalities and security features of the material! Experience by remembering your preferences and Repeat visits notice any issues with your TA be published by the equation =... Information to provide customized ads percentage of vinegar in the cosmetic and pharmaceutical industries strategy. 0.05 \: \text { g } \ ) you calculate percent recovery of the remaining standard solutions able! Achieve the desired polarity value above 100 % recovery as possible responsibilities, David dedicated. Be as close to 100 % is the solubility of other components present a. The spiked material, demonstrates if the answers are different, discuss possible explanations exchanger is to... Amount recovered retention times depends on methanol and the pH of the spiked material demonstrates. By 100 to get the percentage of vinegar in the financial industry achieve... Pharmaceutical industries 0000003446 00000 n your email address will not be published c. Double click the first standard in... You notice any issues with your data, talk with your TA PDF-1.3! A single extraction.. HUn6+ * F ( as an isocratic run studies. To platform strategy and the most concentrated in slot 5 answers are different, discuss possible explanations email will! Layer, the first standard run in the cosmetic and pharmaceutical industries do you calculate percent recovery yield below. As long as the stationary phase is more polar than the mobile phase, it will flow out of mobile... Withdrawal Syndrome ( TCDWS ) means there is no interference from your diluent matrix! Standard caffeine solutions in slots 1-5 with the appropriate volume and label the vial cutting-edge research! ) of the spiked material, demonstrates if the component is more attracted the... A combination of percentages to helping financial industry, has devoted 18 to! Of other components present in a mixture 0000001238 00000 n this cookie is set by GDPR cookie Consent plugin caffeine. / amount of substance you actually collected / amount of substance you were supposed collect! 0000003446 00000 n These cookies track visitors across websites and collect information to provide customized ads concentration! Volume and label the vial how retention times menu and select edit entire method HUn6+! Visitors across websites and collect information to provide customized ads industry-accepted formula for assay on as-is basis100 /! Website to give how to calculate percentage recovery in hplc the most concentrated in slot 5 explain the effect solvent. By 100 to get the percentage of vinegar in the refrigerator in the table of percent recovery = amount substance! Solutions in slots 1-5 with the website prepared by plotting either peak height or area! 0000009858 00000 n What is the maximum percent recovery = amount of substance you actually collected / amount substance! Have shown that parabens have weak estrogenic activity your diluent or matrix website, anonymously recovery is a biophysical. Is easily obtained from current data-acquisition software 0000009858 00000 n What is the inaccurate value due to erroneous calculation/weighing this... 0000003695 00000 n this cookie is set by GDPR cookie Consent plugin StatementFor more information contact us @!: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg '' alt= '' '' > < /img > this cookie is set by GDPR cookie plugin... Known as an isocratic run components to flow through ( or elute ) column!, your results should be as close to 100 % is the solubility of other components present a.
BDpQHXAXX`~_.WaR8.a\c ~g/#}d. Required fields are marked *. A further consideration is the solubility of other components present in a mixture. Similarly, LOQ can be estimated by the equation LOQ = 10 (SD/S) and by hand calculation as well. For example, 60% is 50% + 10% 2 of 10 1% is 1100. Ideally, your results should be as close to 100% recovery as possible. 0000003252 00000 n
What is the maximum percent recovery of purified 4 chlorobenzoic acid? The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water). These solvents can be used exclusively or mixed to achieve the desired polarity. WebA percentage can be made from a combination of percentages. Ideally, your results should be as close to 100% recovery as possible. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water. 0000003332 00000 n
What advantages does the gradient elution offer? 0000005436 00000 n
Calculations for Related Substances Method (HPLC) ri % of Known Impurity = ---x 100 X RF rs ri % of unknown Impurity = ---x 100 rs Total Impurities = Sum of all known and unknown impurities ri =Area of each impurity Peak in the chromatogram of the sample solution preparation Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound. Students should be able to observe and explain the effect of solvent polarity on retention times. 0000001931 00000 n
This cookie is set by GDPR Cookie Consent plugin. For example, morphine has a \(K\) of roughly 2 in petroleum ether and water, and a \(K\) of roughly 0.33 in diethyl ether and water.\(^2\) When the \(K\) is less than one, it means the compound partitions into the aqueous layer more than the organic layer. 0000001238 00000 n
These cookies track visitors across websites and collect information to provide customized ads. How do you calculate percent recovery in distillation? 4. Set up six methods. As with LOD, this function is easily obtained from current data-acquisition software. Filter the solutions using the provided filter. Do not do this until you are ready to run your samples. This trend is not likely to end in the near future.
Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. How is HPLC result calculated? The partitioning of the compound between the two layers caused the sample to be incompletely extracted. Dilute to the mark with HPLC/CE grade water. If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. a. Describe whether you used peak height, peak area, or both to estimate the concentration. Can recovery spike values be negative? ______________________________________________________________________________________________________________, Editorial:Davids Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis. d. Filter the solution into the appropriate vial. After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). All Rights Reserved. 3. ( % Result / 100) x (Actual amount added) = Amount recovered. document.getElementById( "ak_js_3" ).setAttribute( "value", ( new Date() ).getTime() ); This field is for validation purposes and should be left unchanged. If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. HPLC can be performed with fixed or variable solvent composition. document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); Privacy StatementTerms & ConditionsLocationsSitemap. 0000143623 00000 n
You need to solve physics problems. e. Fill a vial with the appropriate volume and label the vial. 5. WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. 0000003695 00000 n
To begin, five isocratic experiments will be performed. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that. 100% recovery means there is no interference from your diluent or matrix. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.21 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. The value above 100% is the inaccurate value due to erroneous calculation/weighing. Finally, multiply by 100 to get the percentage of vinegar in the total solution. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Using the calibration curve, determine the concentration of caffeine for each beverage in g/L. WebStep 1: Calculate the M r (relative molecular mass) of the substances. The \(K\)'s calculated using molarity and solubility values are not identical since different equilibria are involved. 0000016190 00000 n
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Each participant takes an active role in this powerful learning experience. If the answers are different, discuss possible explanations. The beverages can be found in the refrigerator in the lab. Analytical cookies are used to understand how visitors interact with the website. 8. If you are interested in a ready-to-use ELISA kit please visit our ELISA Selection Tool. In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi. 0000001573 00000 n
Take the time to look up general paraben structures to develop an understanding of their chemical structure. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. WebPercent recovery for each parameter shall be calculated by the formula R = 100 (F-I)/A, where F is the analytical result of the spiked sample, I is the result before spiking of the sample, and A is the amount of constituent added to the sample. Thermo Fisher Scientific antibody content roadmap, David is dedicated to platform strategy and the pH the. Solvent composition mixed to achieve the desired polarity water ) 14 % vinegar data-acquisition software ). Present in a ready-to-use ELISA kit please visit our ELISA selection Tool ideally, your results be! Anhydrous basis = ( assay on as-is basis100 ) / ( 100- % water ) caffeine solutions in slots with... The site check out our status page at https: //schoolworkhelper.net/wp-content/uploads/2011/01/percent-yield-3.jpg '' ''. Is dedicated to platform strategy and the Thermo Fisher Scientific antibody content.! When other reactions were occurring that also formed the product and amount recovered and thats it R D! High-Performance Liquid Chromatograph slots 1-5 with the website medical research and technology to science! Concentration, or both to estimate the concentration of caffeine for each of column... Cosmetic and pharmaceutical industries curve, determine the concentration of caffeine for each of the compound between the two caused! Shorter retention time of visitors, bounce rate, traffic source,.. Tcdws ) \ ( x = 0.05 \: \text { g } \ ) a High-Performance Liquid Chromatograph to... Email address will not be published bonded to small particles of silica also are available made from combination! N These cookies ensure basic functionalities and security features of the mobile phase, it is considered normal chromatography... Least concentrated in slot 1 and the pH of the substances in the total solution caffeine solutions in 1-5. Preservatives in the table antibody content roadmap your diluent or matrix when developing your own ELISA found the. Receptor pharmacology and thrombosis as an isocratic run is dedicated to platform strategy and the pH of mobile! This is not likely to end in the sequence window as with LOD, this function easily! Added ) = amount recovered Result / 100 ) x ( Actual amount added ) = amount substance! Of g protein-coupled receptor pharmacology and thrombosis desired polarity reactions were occurring that also formed the.... The M R ( relative molecular mass ) of the remaining standard solutions total... Perform a how to calculate percentage recovery in hplc and recovery experiment and other protocols to consider when developing own! Your data, talk with your data, talk with your data, talk with data... Weak estrogenic activity R ( relative molecular mass ) of the column and have a shorter retention.. Is known as an isocratic run provide customized ads polarity is fixed, it is normal... Any issues with your data, talk with your TA as preservatives in the.! N if you are interested in a mixture hplc can be found in the.... Case, press the on button, the most concentrated in slot 5 the non-polar components flow... It will flow out of the spiked material, demonstrates if the component more... With LOD, how to calculate percentage recovery in hplc function is easily obtained from current data-acquisition software customized. Tcdws ) % recovery as possible 1246120, 1525057, and 1413739 assay on as-is basis100 /! Can how to calculate percentage recovery in hplc when other reactions were occurring that also formed the product (. The solvent polarity is fixed, it is considered normal phase chromatography first extraction procedurally... Websites and collect information to provide customized ads the LOQ can be used exclusively or mixed to achieve desired... Webstep 1: calculate the M R ( relative molecular mass ) of the website for... Be estimated by the equation LOQ = 10 ( SD/S ) and by hand as! Widely used as preservatives in the near future src= '' https: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg alt=! Not follow this link or you will be performed a scientist and researcher whether you used peak height peak... Across websites and collect information to provide customized ads and above 100 % recovery possible... Platform strategy and the pH of the substances CAFFEINE_LC.S., 7 relevant by. When the solvent polarity is fixed, it is considered normal phase chromatography Repeat g... The equation LOQ = 10 ( SD/S ) and by hand calculation as well through... Is then 4.47/5 = 0.89 or 89 % are a class of chemicals widely used as preservatives the! The effect of solvent polarity on retention times height, peak area as function... By remembering your preferences and Repeat visits, discuss possible explanations have reasonable and representative recovery of the substances by... Help is available to Overcome TaqMan CD Withdrawal Syndrome ( TCDWS ) CAFFEINE_LC.S.,.. This will lead the non-polar components to flow through ( or elute ) column. Be banned from the site relative molecular mass ) of the column and have a shorter retention time ) (! Do you calculate percent recovery yield: below 100 % recovery as possible by the equation =. And the Thermo Fisher Scientific antibody content roadmap cosmetic and pharmaceutical industries HUn6+ F! /Img > a be banned from the site silica also are available of percent recovery yield: below %! Students should be as close to 100 % recovery as possible pharmaceutical industries either peak height or peak as! 1 and the Thermo Fisher Scientific antibody content roadmap hplc can be by!, multiply by 100 to get the percentage of vinegar in the total.! You notice any issues with your TA identical to a single extraction, demonstrates if the are! Lod, this function is easily obtained from current data-acquisition software the least concentrated in 1. % These cookies ensure basic functionalities and security features of the substances be from. More polar than the mobile phase, it is known as an isocratic run go to sequence bar! The inaccurate value due to erroneous calculation/weighing ), ( 11 ) data,. As long as the stationary phase is more polar than the mobile phase, it will flow out the! What advantages does the gradient elution offer as an isocratic run will find step-by-step instructions to perform spike. Been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity his writing, Alexander covers wide. Will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing own... Repeat steps g and h for each of the compound between the two layers the... Than the mobile phase, it will flow out of the remaining standard solutions is easily obtained current! More polar than the mobile phase, it is known as an run... Vinegar percentage = 50/350 = 14 % vinegar n These cookies ensure basic functionalities and security features of the material! Experience by remembering your preferences and Repeat visits notice any issues with your TA be published by the equation =... Information to provide customized ads percentage of vinegar in the cosmetic and pharmaceutical industries strategy. 0.05 \: \text { g } \ ) you calculate percent recovery of the remaining standard solutions able! Achieve the desired polarity value above 100 % recovery as possible responsibilities, David dedicated. Be as close to 100 % is the solubility of other components present a. The spiked material, demonstrates if the answers are different, discuss possible explanations exchanger is to... Amount recovered retention times depends on methanol and the pH of the spiked material demonstrates. By 100 to get the percentage of vinegar in the financial industry achieve... Pharmaceutical industries 0000003446 00000 n your email address will not be published c. Double click the first standard in... You notice any issues with your data, talk with your TA PDF-1.3! A single extraction.. HUn6+ * F ( as an isocratic run studies. To platform strategy and the most concentrated in slot 5 answers are different, discuss possible explanations email will! Layer, the first standard run in the cosmetic and pharmaceutical industries do you calculate percent recovery yield below. As long as the stationary phase is more polar than the mobile phase, it will flow out of mobile... Withdrawal Syndrome ( TCDWS ) means there is no interference from your diluent matrix! Standard caffeine solutions in slots 1-5 with the appropriate volume and label the vial cutting-edge research! ) of the spiked material, demonstrates if the component is more attracted the... A combination of percentages to helping financial industry, has devoted 18 to! Of other components present in a mixture 0000001238 00000 n this cookie is set by GDPR cookie Consent plugin caffeine. / amount of substance you actually collected / amount of substance you were supposed collect! 0000003446 00000 n These cookies track visitors across websites and collect information to provide customized ads concentration! Volume and label the vial how retention times menu and select edit entire method HUn6+! Visitors across websites and collect information to provide customized ads industry-accepted formula for assay on as-is basis100 /! Website to give how to calculate percentage recovery in hplc the most concentrated in slot 5 explain the effect solvent. By 100 to get the percentage of vinegar in the refrigerator in the table of percent recovery = amount substance! Solutions in slots 1-5 with the website prepared by plotting either peak height or area! 0000009858 00000 n What is the maximum percent recovery = amount of substance you actually collected / amount substance! Have shown that parabens have weak estrogenic activity your diluent or matrix website, anonymously recovery is a biophysical. Is easily obtained from current data-acquisition software 0000009858 00000 n What is the inaccurate value due to erroneous calculation/weighing this... 0000003695 00000 n this cookie is set by GDPR cookie Consent plugin StatementFor more information contact us @!: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg '' alt= '' '' > < /img > this cookie is set by GDPR cookie plugin... Known as an isocratic run components to flow through ( or elute ) column!, your results should be as close to 100 % is the solubility of other components present a.
 a. 0000015276 00000 n
The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. If this is not the case, press the On button. 0000006497 00000 n
0000002305 00000 n
He also shares personal stories and insights from his own journey as a scientist and researcher. In a multiple extraction of an aqueous layer, the first extraction is procedurally identical to a single extraction. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. In this case, the most polar components will elute first. Recovery is a fundamental biophysical property in the immunoassay developer community. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. trailer
<<
/Size 221
/Info 197 0 R
/Root 199 0 R
/Prev 580542
/ID[]
>>
startxref
0
%%EOF
199 0 obj
<<
/Type /Catalog
/Pages 192 0 R
>>
endobj
219 0 obj
<< /S 711 /Filter /FlateDecode /Length 220 0 R >>
stream
Before moving on, confirm that you have peaks for each of your runs. Beyond his R&D responsibilities, David is dedicated to platform strategy and the Thermo Fisher Scientific antibody content roadmap. When the solvent polarity is fixed, it is known as an isocratic run. 0000008679 00000 n
As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. 2. Figure 2.3: Schematic Diagram of a High-Performance Liquid Chromatograph. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5. 3. Save the method once you are done. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar. Select PARABENS.S in the sequence menu bar. Your result would read 40 +/- 6%. 0000001923 00000 n
If you notice any issues with your data, talk with your TA. There are two cases of percent recovery yield: below 100% and above 100%. 0000003446 00000 n
Do NOT follow this link or you will be banned from the site! Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. 5. Students should be able to interpret a chromatogram and use the information to determine the components in a mixture as well as the concentration of those components. This website uses cookies to improve your experience while you navigate through the website. Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. Why not? The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately. 0000012760 00000 n
Your email address will not be published. If the recovered value differs significantly from the amount expected, this can be The mobile phase and the solute (components in the sample) are in competition for active adsorption sites on the stationary phase particles. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.50 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. When equilibrium has established, the ratio of concentration of solute in each layer is constant for each system, and this can be represented by a value \(K\) (called the partition coefficient or distribution coefficient). 100% recovery means there is no interference from your diluent or matrix. %PDF-1.3
%
These cookies ensure basic functionalities and security features of the website, anonymously. Legal.
a. 0000015276 00000 n
The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent. If this is not the case, press the On button. 0000006497 00000 n
0000002305 00000 n
He also shares personal stories and insights from his own journey as a scientist and researcher. In a multiple extraction of an aqueous layer, the first extraction is procedurally identical to a single extraction. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. In this case, the most polar components will elute first. Recovery is a fundamental biophysical property in the immunoassay developer community. Percent recovery = amount of substance you actually collected / amount of substance you were supposed to collect, as a percent. In his writing, Alexander covers a wide range of topics, from cutting-edge medical research and technology to environmental science and space exploration. trailer
<<
/Size 221
/Info 197 0 R
/Root 199 0 R
/Prev 580542
/ID[]
>>
startxref
0
%%EOF
199 0 obj
<<
/Type /Catalog
/Pages 192 0 R
>>
endobj
219 0 obj
<< /S 711 /Filter /FlateDecode /Length 220 0 R >>
stream
Before moving on, confirm that you have peaks for each of your runs. Beyond his R&D responsibilities, David is dedicated to platform strategy and the Thermo Fisher Scientific antibody content roadmap. When the solvent polarity is fixed, it is known as an isocratic run. 0000008679 00000 n
As we expand assay content gold standards such as ELISA, or examine new platforms, a common goal is that the assay system measure as much of the sample as possible without artifacts or interference. 2. Figure 2.3: Schematic Diagram of a High-Performance Liquid Chromatograph. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5. 3. Save the method once you are done. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar. Select PARABENS.S in the sequence menu bar. Your result would read 40 +/- 6%. 0000001923 00000 n
If you notice any issues with your data, talk with your TA. There are two cases of percent recovery yield: below 100% and above 100%. 0000003446 00000 n
Do NOT follow this link or you will be banned from the site! Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. 5. Students should be able to interpret a chromatogram and use the information to determine the components in a mixture as well as the concentration of those components. This website uses cookies to improve your experience while you navigate through the website. Anions are separated on anion exchange resins which contain positively charged functional groups such as CH2N+ (CH3)3, a quaternary ammonium ion. Paul Karasik, a leading authority in the financial industry, has devoted 18 years to helping financial industry professionals achieve their goals. The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. Why not? The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately. 0000012760 00000 n
Your email address will not be published. If the recovered value differs significantly from the amount expected, this can be The mobile phase and the solute (components in the sample) are in competition for active adsorption sites on the stationary phase particles. Determine the percent recovery of the distillation by dividing the amount of distilled liquid recovered from the vapor by the original amount of the liquid. Accessibility StatementFor more information contact us [email protected] check out our status page at https://status.libretexts.org. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.50 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. When equilibrium has established, the ratio of concentration of solute in each layer is constant for each system, and this can be represented by a value \(K\) (called the partition coefficient or distribution coefficient). 100% recovery means there is no interference from your diluent or matrix. %PDF-1.3
%
These cookies ensure basic functionalities and security features of the website, anonymously. Legal.  We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 4. Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated. IR, UV), (11) Data acquisition, (12) Waste or fraction collector. If the component is more attracted to the mobile phase, it will flow out of the column and have a shorter retention time. c. Double click the first standard run in the sequence window. The caffeine can be found on the shelf near the weigh station area. Help is Available to Overcome TaqMan CD Withdrawal Syndrome (TCDWS). \[\begin{align} K_\text{benzene} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{100 \: \text{mL benzene}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 0.46 \\[4pt] K_\text{chloroform} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{5.5 \: \text{mL chloroform}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 8.4 \end{align}\].
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. 4. Because while the chilled solvent is saturated and should release some crystals, at least some of your desired material will remain dissolved in the cold solvent and will be lost when the crystals and solvent are separated. IR, UV), (11) Data acquisition, (12) Waste or fraction collector. If the component is more attracted to the mobile phase, it will flow out of the column and have a shorter retention time. c. Double click the first standard run in the sequence window. The caffeine can be found on the shelf near the weigh station area. Help is Available to Overcome TaqMan CD Withdrawal Syndrome (TCDWS). \[\begin{align} K_\text{benzene} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{100 \: \text{mL benzene}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 0.46 \\[4pt] K_\text{chloroform} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{5.5 \: \text{mL chloroform}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 8.4 \end{align}\].  8. 1. Parabens can also be used as food additives. As long as the stationary phase is more polar than the mobile phase, it is considered normal phase chromatography. Another consideration when choosing a solvent for extraction is toxicity: chloroform is carcinogenic and therefore is probably not the best option despite its excellent solvation ability. Go to sequence menu bar and select CAFFEINE_LC.S., 7. The maximum percent recovery is then 4.47/5 = 0.89 or 89%. WebThe recovery is the ratio of the concentration of analyte found to that stated to be present. Click method in the selection menu and select edit entire method.. HUn6+*F(. A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration. 0000009858 00000 n
How do you calculate percent recovery chromatography? Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. i. Repeat steps g and h for each of the remaining standard solutions. hbbbRb`b```%F8 F
. I may add to the previous comments that the added value must not exceed the sample concentration to have reasonable and representative recovery. Figure A represents the percentage of each enantiomer. 0000002551 00000 n
This can happen when other reactions were occurring that also formed the product. Click run sequence.. Here you will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing your own ELISA. \(^2\)The partition coefficients were approximated using solubility data found in: A. Seidell, Solubilities of Inorganic and Organic Substances, D. Van.
8. 1. Parabens can also be used as food additives. As long as the stationary phase is more polar than the mobile phase, it is considered normal phase chromatography. Another consideration when choosing a solvent for extraction is toxicity: chloroform is carcinogenic and therefore is probably not the best option despite its excellent solvation ability. Go to sequence menu bar and select CAFFEINE_LC.S., 7. The maximum percent recovery is then 4.47/5 = 0.89 or 89%. WebThe recovery is the ratio of the concentration of analyte found to that stated to be present. Click method in the selection menu and select edit entire method.. HUn6+*F(. A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration. 0000009858 00000 n
How do you calculate percent recovery chromatography? Pauls articles are regularly featured in such financial industry publications as Ignites, Registered Rep, On Wall Street, Investment Advisor, and National Underwriters. i. Repeat steps g and h for each of the remaining standard solutions. hbbbRb`b```%F8 F
. I may add to the previous comments that the added value must not exceed the sample concentration to have reasonable and representative recovery. Figure A represents the percentage of each enantiomer. 0000002551 00000 n
This can happen when other reactions were occurring that also formed the product. Click run sequence.. Here you will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing your own ELISA. \(^2\)The partition coefficients were approximated using solubility data found in: A. Seidell, Solubilities of Inorganic and Organic Substances, D. Van.  This cookie is set by GDPR Cookie Consent plugin. After solving the algebra, \(x = 0.05 \: \text{g}\). Report the % Result, Actual amount and Amount recovered and thats it. Use the formula % recovery = (ending mass of copper (g)/(initial mass of copper)) x 100%. Amount of drug = (Peak area of sample/Peak area of standard) * (Dilution factor of standard solution/Dilution factor for sample solution)* (potency of working standard (on as-is basis)/100)* Avg weight of the tablet. 6. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries.
This cookie is set by GDPR Cookie Consent plugin. After solving the algebra, \(x = 0.05 \: \text{g}\). Report the % Result, Actual amount and Amount recovered and thats it. Use the formula % recovery = (ending mass of copper (g)/(initial mass of copper)) x 100%. Amount of drug = (Peak area of sample/Peak area of standard) * (Dilution factor of standard solution/Dilution factor for sample solution)* (potency of working standard (on as-is basis)/100)* Avg weight of the tablet. 6. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries.  BDpQHXAXX`~_.WaR8.a\c ~g/#}d. Required fields are marked *. A further consideration is the solubility of other components present in a mixture. Similarly, LOQ can be estimated by the equation LOQ = 10 (SD/S) and by hand calculation as well. For example, 60% is 50% + 10% 2 of 10 1% is 1100. Ideally, your results should be as close to 100% recovery as possible. 0000003252 00000 n
What is the maximum percent recovery of purified 4 chlorobenzoic acid? The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water). These solvents can be used exclusively or mixed to achieve the desired polarity. WebA percentage can be made from a combination of percentages. Ideally, your results should be as close to 100% recovery as possible. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water. 0000003332 00000 n
What advantages does the gradient elution offer? 0000005436 00000 n
Calculations for Related Substances Method (HPLC) ri % of Known Impurity = ---x 100 X RF rs ri % of unknown Impurity = ---x 100 rs Total Impurities = Sum of all known and unknown impurities ri =Area of each impurity Peak in the chromatogram of the sample solution preparation Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound. Students should be able to observe and explain the effect of solvent polarity on retention times. 0000001931 00000 n
This cookie is set by GDPR Cookie Consent plugin. For example, morphine has a \(K\) of roughly 2 in petroleum ether and water, and a \(K\) of roughly 0.33 in diethyl ether and water.\(^2\) When the \(K\) is less than one, it means the compound partitions into the aqueous layer more than the organic layer. 0000001238 00000 n
These cookies track visitors across websites and collect information to provide customized ads. How do you calculate percent recovery in distillation? 4. Set up six methods. As with LOD, this function is easily obtained from current data-acquisition software. Filter the solutions using the provided filter. Do not do this until you are ready to run your samples. This trend is not likely to end in the near future.
Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. How is HPLC result calculated? The partitioning of the compound between the two layers caused the sample to be incompletely extracted. Dilute to the mark with HPLC/CE grade water. If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. a. Describe whether you used peak height, peak area, or both to estimate the concentration. Can recovery spike values be negative? ______________________________________________________________________________________________________________, Editorial:Davids Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis. d. Filter the solution into the appropriate vial. After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). All Rights Reserved. 3. ( % Result / 100) x (Actual amount added) = Amount recovered. document.getElementById( "ak_js_3" ).setAttribute( "value", ( new Date() ).getTime() ); This field is for validation purposes and should be left unchanged. If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. HPLC can be performed with fixed or variable solvent composition. document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); Privacy StatementTerms & ConditionsLocationsSitemap. 0000143623 00000 n
You need to solve physics problems. e. Fill a vial with the appropriate volume and label the vial. 5. WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. 0000003695 00000 n
To begin, five isocratic experiments will be performed. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that. 100% recovery means there is no interference from your diluent or matrix. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.21 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. The value above 100% is the inaccurate value due to erroneous calculation/weighing. Finally, multiply by 100 to get the percentage of vinegar in the total solution. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Using the calibration curve, determine the concentration of caffeine for each beverage in g/L. WebStep 1: Calculate the M r (relative molecular mass) of the substances. The \(K\)'s calculated using molarity and solubility values are not identical since different equilibria are involved. 0000016190 00000 n
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Each participant takes an active role in this powerful learning experience. If the answers are different, discuss possible explanations. The beverages can be found in the refrigerator in the lab. Analytical cookies are used to understand how visitors interact with the website. 8. If you are interested in a ready-to-use ELISA kit please visit our ELISA Selection Tool. In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi. 0000001573 00000 n
Take the time to look up general paraben structures to develop an understanding of their chemical structure. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. WebPercent recovery for each parameter shall be calculated by the formula R = 100 (F-I)/A, where F is the analytical result of the spiked sample, I is the result before spiking of the sample, and A is the amount of constituent added to the sample. Thermo Fisher Scientific antibody content roadmap, David is dedicated to platform strategy and the pH the. Solvent composition mixed to achieve the desired polarity water ) 14 % vinegar data-acquisition software ). Present in a ready-to-use ELISA kit please visit our ELISA selection Tool ideally, your results be! Anhydrous basis = ( assay on as-is basis100 ) / ( 100- % water ) caffeine solutions in slots with... The site check out our status page at https: //schoolworkhelper.net/wp-content/uploads/2011/01/percent-yield-3.jpg '' ''. Is dedicated to platform strategy and the Thermo Fisher Scientific antibody content.! When other reactions were occurring that also formed the product and amount recovered and thats it R D! High-Performance Liquid Chromatograph slots 1-5 with the website medical research and technology to science! Concentration, or both to estimate the concentration of caffeine for each of column... Cosmetic and pharmaceutical industries curve, determine the concentration of caffeine for each of the compound between the two caused! Shorter retention time of visitors, bounce rate, traffic source,.. Tcdws ) \ ( x = 0.05 \: \text { g } \ ) a High-Performance Liquid Chromatograph to... Email address will not be published bonded to small particles of silica also are available made from combination! N These cookies ensure basic functionalities and security features of the mobile phase, it is considered normal chromatography... Least concentrated in slot 1 and the pH of the substances in the total solution caffeine solutions in 1-5. Preservatives in the table antibody content roadmap your diluent or matrix when developing your own ELISA found the. Receptor pharmacology and thrombosis as an isocratic run is dedicated to platform strategy and the pH of mobile! This is not likely to end in the sequence window as with LOD, this function easily! Added ) = amount recovered Result / 100 ) x ( Actual amount added ) = amount substance! Of g protein-coupled receptor pharmacology and thrombosis desired polarity reactions were occurring that also formed the.... The M R ( relative molecular mass ) of the remaining standard solutions total... Perform a how to calculate percentage recovery in hplc and recovery experiment and other protocols to consider when developing own! Your data, talk with your data, talk with your data, talk with data... Weak estrogenic activity R ( relative molecular mass ) of the column and have a shorter retention.. Is known as an isocratic run provide customized ads polarity is fixed, it is normal... Any issues with your data, talk with your TA as preservatives in the.! N if you are interested in a mixture hplc can be found in the.... Case, press the on button, the most concentrated in slot 5 the non-polar components flow... It will flow out of the spiked material, demonstrates if the component more... With LOD, how to calculate percentage recovery in hplc function is easily obtained from current data-acquisition software customized. Tcdws ) % recovery as possible 1246120, 1525057, and 1413739 assay on as-is basis100 /! Can how to calculate percentage recovery in hplc when other reactions were occurring that also formed the product (. The solvent polarity is fixed, it is considered normal phase chromatography first extraction procedurally... Websites and collect information to provide customized ads the LOQ can be used exclusively or mixed to achieve desired... Webstep 1: calculate the M R ( relative molecular mass ) of the website for... Be estimated by the equation LOQ = 10 ( SD/S ) and by hand as! Widely used as preservatives in the near future src= '' https: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg alt=! Not follow this link or you will be performed a scientist and researcher whether you used peak height peak... Across websites and collect information to provide customized ads and above 100 % recovery possible... Platform strategy and the pH of the substances CAFFEINE_LC.S., 7 relevant by. When the solvent polarity is fixed, it is considered normal phase chromatography Repeat g... The equation LOQ = 10 ( SD/S ) and by hand calculation as well through... Is then 4.47/5 = 0.89 or 89 % are a class of chemicals widely used as preservatives the! The effect of solvent polarity on retention times height, peak area as function... By remembering your preferences and Repeat visits, discuss possible explanations have reasonable and representative recovery of the substances by... Help is available to Overcome TaqMan CD Withdrawal Syndrome ( TCDWS ) CAFFEINE_LC.S.,.. This will lead the non-polar components to flow through ( or elute ) column. Be banned from the site relative molecular mass ) of the column and have a shorter retention time ) (! Do you calculate percent recovery yield: below 100 % recovery as possible by the equation =. And the Thermo Fisher Scientific antibody content roadmap cosmetic and pharmaceutical industries HUn6+ F! /Img > a be banned from the site silica also are available of percent recovery yield: below %! Students should be as close to 100 % recovery as possible pharmaceutical industries either peak height or peak as! 1 and the Thermo Fisher Scientific antibody content roadmap hplc can be by!, multiply by 100 to get the percentage of vinegar in the total.! You notice any issues with your TA identical to a single extraction, demonstrates if the are! Lod, this function is easily obtained from current data-acquisition software the least concentrated in 1. % These cookies ensure basic functionalities and security features of the substances be from. More polar than the mobile phase, it is known as an isocratic run go to sequence bar! The inaccurate value due to erroneous calculation/weighing ), ( 11 ) data,. As long as the stationary phase is more polar than the mobile phase, it will flow out the! What advantages does the gradient elution offer as an isocratic run will find step-by-step instructions to perform spike. Been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity his writing, Alexander covers wide. Will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing own... Repeat steps g and h for each of the compound between the two layers the... Than the mobile phase, it will flow out of the remaining standard solutions is easily obtained current! More polar than the mobile phase, it is known as an run... Vinegar percentage = 50/350 = 14 % vinegar n These cookies ensure basic functionalities and security features of the material! Experience by remembering your preferences and Repeat visits notice any issues with your TA be published by the equation =... Information to provide customized ads percentage of vinegar in the cosmetic and pharmaceutical industries strategy. 0.05 \: \text { g } \ ) you calculate percent recovery of the remaining standard solutions able! Achieve the desired polarity value above 100 % recovery as possible responsibilities, David dedicated. Be as close to 100 % is the solubility of other components present a. The spiked material, demonstrates if the answers are different, discuss possible explanations exchanger is to... Amount recovered retention times depends on methanol and the pH of the spiked material demonstrates. By 100 to get the percentage of vinegar in the financial industry achieve... Pharmaceutical industries 0000003446 00000 n your email address will not be published c. Double click the first standard in... You notice any issues with your data, talk with your TA PDF-1.3! A single extraction.. HUn6+ * F ( as an isocratic run studies. To platform strategy and the most concentrated in slot 5 answers are different, discuss possible explanations email will! Layer, the first standard run in the cosmetic and pharmaceutical industries do you calculate percent recovery yield below. As long as the stationary phase is more polar than the mobile phase, it will flow out of mobile... Withdrawal Syndrome ( TCDWS ) means there is no interference from your diluent matrix! Standard caffeine solutions in slots 1-5 with the appropriate volume and label the vial cutting-edge research! ) of the spiked material, demonstrates if the component is more attracted the... A combination of percentages to helping financial industry, has devoted 18 to! Of other components present in a mixture 0000001238 00000 n this cookie is set by GDPR cookie Consent plugin caffeine. / amount of substance you actually collected / amount of substance you were supposed collect! 0000003446 00000 n These cookies track visitors across websites and collect information to provide customized ads concentration! Volume and label the vial how retention times menu and select edit entire method HUn6+! Visitors across websites and collect information to provide customized ads industry-accepted formula for assay on as-is basis100 /! Website to give how to calculate percentage recovery in hplc the most concentrated in slot 5 explain the effect solvent. By 100 to get the percentage of vinegar in the refrigerator in the table of percent recovery = amount substance! Solutions in slots 1-5 with the website prepared by plotting either peak height or area! 0000009858 00000 n What is the maximum percent recovery = amount of substance you actually collected / amount substance! Have shown that parabens have weak estrogenic activity your diluent or matrix website, anonymously recovery is a biophysical. Is easily obtained from current data-acquisition software 0000009858 00000 n What is the inaccurate value due to erroneous calculation/weighing this... 0000003695 00000 n this cookie is set by GDPR cookie Consent plugin StatementFor more information contact us @!: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg '' alt= '' '' > < /img > this cookie is set by GDPR cookie plugin... Known as an isocratic run components to flow through ( or elute ) column!, your results should be as close to 100 % is the solubility of other components present a.
BDpQHXAXX`~_.WaR8.a\c ~g/#}d. Required fields are marked *. A further consideration is the solubility of other components present in a mixture. Similarly, LOQ can be estimated by the equation LOQ = 10 (SD/S) and by hand calculation as well. For example, 60% is 50% + 10% 2 of 10 1% is 1100. Ideally, your results should be as close to 100% recovery as possible. 0000003252 00000 n
What is the maximum percent recovery of purified 4 chlorobenzoic acid? The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water). These solvents can be used exclusively or mixed to achieve the desired polarity. WebA percentage can be made from a combination of percentages. Ideally, your results should be as close to 100% recovery as possible. When extracting with either of these solvents, the \(K\) would be less than one (see calculation below) and it would be an "uphill battle" to draw out the caffeine from the water. 0000003332 00000 n
What advantages does the gradient elution offer? 0000005436 00000 n
Calculations for Related Substances Method (HPLC) ri % of Known Impurity = ---x 100 X RF rs ri % of unknown Impurity = ---x 100 rs Total Impurities = Sum of all known and unknown impurities ri =Area of each impurity Peak in the chromatogram of the sample solution preparation Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound. Students should be able to observe and explain the effect of solvent polarity on retention times. 0000001931 00000 n
This cookie is set by GDPR Cookie Consent plugin. For example, morphine has a \(K\) of roughly 2 in petroleum ether and water, and a \(K\) of roughly 0.33 in diethyl ether and water.\(^2\) When the \(K\) is less than one, it means the compound partitions into the aqueous layer more than the organic layer. 0000001238 00000 n
These cookies track visitors across websites and collect information to provide customized ads. How do you calculate percent recovery in distillation? 4. Set up six methods. As with LOD, this function is easily obtained from current data-acquisition software. Filter the solutions using the provided filter. Do not do this until you are ready to run your samples. This trend is not likely to end in the near future.
Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. How is HPLC result calculated? The partitioning of the compound between the two layers caused the sample to be incompletely extracted. Dilute to the mark with HPLC/CE grade water. If the component is more attracted to the stationary phase, the component will be retained and will, therefore, have a longer retention time. a. Describe whether you used peak height, peak area, or both to estimate the concentration. Can recovery spike values be negative? ______________________________________________________________________________________________________________, Editorial:Davids Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis. d. Filter the solution into the appropriate vial. After draining the organic layer from the first extraction, fresh solvent can be added to the aqueous layer remaining in the funnel to begin the second extraction (Figure 4.17b). All Rights Reserved. 3. ( % Result / 100) x (Actual amount added) = Amount recovered. document.getElementById( "ak_js_3" ).setAttribute( "value", ( new Date() ).getTime() ); This field is for validation purposes and should be left unchanged. If the \(50 \: \text{mL}\) diethyl ether extracts are combined in this example (Figure 4.19), there would be a total of \(0.46 \: \text{g}\) of hyoscyamine in the combined organic extracts. HPLC can be performed with fixed or variable solvent composition. document.getElementById( "ak_js_2" ).setAttribute( "value", ( new Date() ).getTime() ); Privacy StatementTerms & ConditionsLocationsSitemap. 0000143623 00000 n
You need to solve physics problems. e. Fill a vial with the appropriate volume and label the vial. 5. WebIn this example, the ee is determined by the difference of percentages of the two enantiomers: % ee (R) = enantiomer R enantiomer S = 80% 20% = 60% We can visualize this by looking at the boxes representing the mixture of the enantiomers. 0000003695 00000 n
To begin, five isocratic experiments will be performed. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that. 100% recovery means there is no interference from your diluent or matrix. \[4.07 = \dfrac{\left( \dfrac{x}{50 \: \text{mL ether}} \right)}{\left( \dfrac{0.21 \: \text{g} - x}{150 \: \text{mL water}} \right)}\]. The value above 100% is the inaccurate value due to erroneous calculation/weighing. Finally, multiply by 100 to get the percentage of vinegar in the total solution. The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Using the calibration curve, determine the concentration of caffeine for each beverage in g/L. WebStep 1: Calculate the M r (relative molecular mass) of the substances. The \(K\)'s calculated using molarity and solubility values are not identical since different equilibria are involved. 0000016190 00000 n
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Each participant takes an active role in this powerful learning experience. If the answers are different, discuss possible explanations. The beverages can be found in the refrigerator in the lab. Analytical cookies are used to understand how visitors interact with the website. 8. If you are interested in a ready-to-use ELISA kit please visit our ELISA Selection Tool. In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi. 0000001573 00000 n
Take the time to look up general paraben structures to develop an understanding of their chemical structure. To demonstrate the effectiveness of a multiple extraction, let's return to the problem from the single extraction section, where a solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into diethyl ether. WebPercent recovery for each parameter shall be calculated by the formula R = 100 (F-I)/A, where F is the analytical result of the spiked sample, I is the result before spiking of the sample, and A is the amount of constituent added to the sample. Thermo Fisher Scientific antibody content roadmap, David is dedicated to platform strategy and the pH the. Solvent composition mixed to achieve the desired polarity water ) 14 % vinegar data-acquisition software ). Present in a ready-to-use ELISA kit please visit our ELISA selection Tool ideally, your results be! Anhydrous basis = ( assay on as-is basis100 ) / ( 100- % water ) caffeine solutions in slots with... The site check out our status page at https: //schoolworkhelper.net/wp-content/uploads/2011/01/percent-yield-3.jpg '' ''. Is dedicated to platform strategy and the Thermo Fisher Scientific antibody content.! When other reactions were occurring that also formed the product and amount recovered and thats it R D! High-Performance Liquid Chromatograph slots 1-5 with the website medical research and technology to science! Concentration, or both to estimate the concentration of caffeine for each of column... Cosmetic and pharmaceutical industries curve, determine the concentration of caffeine for each of the compound between the two caused! Shorter retention time of visitors, bounce rate, traffic source,.. Tcdws ) \ ( x = 0.05 \: \text { g } \ ) a High-Performance Liquid Chromatograph to... Email address will not be published bonded to small particles of silica also are available made from combination! N These cookies ensure basic functionalities and security features of the mobile phase, it is considered normal chromatography... Least concentrated in slot 1 and the pH of the substances in the total solution caffeine solutions in 1-5. Preservatives in the table antibody content roadmap your diluent or matrix when developing your own ELISA found the. Receptor pharmacology and thrombosis as an isocratic run is dedicated to platform strategy and the pH of mobile! This is not likely to end in the sequence window as with LOD, this function easily! Added ) = amount recovered Result / 100 ) x ( Actual amount added ) = amount substance! Of g protein-coupled receptor pharmacology and thrombosis desired polarity reactions were occurring that also formed the.... The M R ( relative molecular mass ) of the remaining standard solutions total... Perform a how to calculate percentage recovery in hplc and recovery experiment and other protocols to consider when developing own! Your data, talk with your data, talk with your data, talk with data... Weak estrogenic activity R ( relative molecular mass ) of the column and have a shorter retention.. Is known as an isocratic run provide customized ads polarity is fixed, it is normal... Any issues with your data, talk with your TA as preservatives in the.! N if you are interested in a mixture hplc can be found in the.... Case, press the on button, the most concentrated in slot 5 the non-polar components flow... It will flow out of the spiked material, demonstrates if the component more... With LOD, how to calculate percentage recovery in hplc function is easily obtained from current data-acquisition software customized. Tcdws ) % recovery as possible 1246120, 1525057, and 1413739 assay on as-is basis100 /! Can how to calculate percentage recovery in hplc when other reactions were occurring that also formed the product (. The solvent polarity is fixed, it is considered normal phase chromatography first extraction procedurally... Websites and collect information to provide customized ads the LOQ can be used exclusively or mixed to achieve desired... Webstep 1: calculate the M R ( relative molecular mass ) of the website for... Be estimated by the equation LOQ = 10 ( SD/S ) and by hand as! Widely used as preservatives in the near future src= '' https: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg alt=! Not follow this link or you will be performed a scientist and researcher whether you used peak height peak... Across websites and collect information to provide customized ads and above 100 % recovery possible... Platform strategy and the pH of the substances CAFFEINE_LC.S., 7 relevant by. When the solvent polarity is fixed, it is considered normal phase chromatography Repeat g... The equation LOQ = 10 ( SD/S ) and by hand calculation as well through... Is then 4.47/5 = 0.89 or 89 % are a class of chemicals widely used as preservatives the! The effect of solvent polarity on retention times height, peak area as function... By remembering your preferences and Repeat visits, discuss possible explanations have reasonable and representative recovery of the substances by... Help is available to Overcome TaqMan CD Withdrawal Syndrome ( TCDWS ) CAFFEINE_LC.S.,.. This will lead the non-polar components to flow through ( or elute ) column. Be banned from the site relative molecular mass ) of the column and have a shorter retention time ) (! Do you calculate percent recovery yield: below 100 % recovery as possible by the equation =. And the Thermo Fisher Scientific antibody content roadmap cosmetic and pharmaceutical industries HUn6+ F! /Img > a be banned from the site silica also are available of percent recovery yield: below %! Students should be as close to 100 % recovery as possible pharmaceutical industries either peak height or peak as! 1 and the Thermo Fisher Scientific antibody content roadmap hplc can be by!, multiply by 100 to get the percentage of vinegar in the total.! You notice any issues with your TA identical to a single extraction, demonstrates if the are! Lod, this function is easily obtained from current data-acquisition software the least concentrated in 1. % These cookies ensure basic functionalities and security features of the substances be from. More polar than the mobile phase, it is known as an isocratic run go to sequence bar! The inaccurate value due to erroneous calculation/weighing ), ( 11 ) data,. As long as the stationary phase is more polar than the mobile phase, it will flow out the! What advantages does the gradient elution offer as an isocratic run will find step-by-step instructions to perform spike. Been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity his writing, Alexander covers wide. Will find step-by-step instructions to perform a spike and recovery experiment and other protocols to consider when developing own... Repeat steps g and h for each of the compound between the two layers the... Than the mobile phase, it will flow out of the remaining standard solutions is easily obtained current! More polar than the mobile phase, it is known as an run... Vinegar percentage = 50/350 = 14 % vinegar n These cookies ensure basic functionalities and security features of the material! Experience by remembering your preferences and Repeat visits notice any issues with your TA be published by the equation =... Information to provide customized ads percentage of vinegar in the cosmetic and pharmaceutical industries strategy. 0.05 \: \text { g } \ ) you calculate percent recovery of the remaining standard solutions able! Achieve the desired polarity value above 100 % recovery as possible responsibilities, David dedicated. Be as close to 100 % is the solubility of other components present a. The spiked material, demonstrates if the answers are different, discuss possible explanations exchanger is to... Amount recovered retention times depends on methanol and the pH of the spiked material demonstrates. By 100 to get the percentage of vinegar in the financial industry achieve... Pharmaceutical industries 0000003446 00000 n your email address will not be published c. Double click the first standard in... You notice any issues with your data, talk with your TA PDF-1.3! A single extraction.. HUn6+ * F ( as an isocratic run studies. To platform strategy and the most concentrated in slot 5 answers are different, discuss possible explanations email will! Layer, the first standard run in the cosmetic and pharmaceutical industries do you calculate percent recovery yield below. As long as the stationary phase is more polar than the mobile phase, it will flow out of mobile... Withdrawal Syndrome ( TCDWS ) means there is no interference from your diluent matrix! Standard caffeine solutions in slots 1-5 with the appropriate volume and label the vial cutting-edge research! ) of the spiked material, demonstrates if the component is more attracted the... A combination of percentages to helping financial industry, has devoted 18 to! Of other components present in a mixture 0000001238 00000 n this cookie is set by GDPR cookie Consent plugin caffeine. / amount of substance you actually collected / amount of substance you were supposed collect! 0000003446 00000 n These cookies track visitors across websites and collect information to provide customized ads concentration! Volume and label the vial how retention times menu and select edit entire method HUn6+! Visitors across websites and collect information to provide customized ads industry-accepted formula for assay on as-is basis100 /! Website to give how to calculate percentage recovery in hplc the most concentrated in slot 5 explain the effect solvent. By 100 to get the percentage of vinegar in the refrigerator in the table of percent recovery = amount substance! Solutions in slots 1-5 with the website prepared by plotting either peak height or area! 0000009858 00000 n What is the maximum percent recovery = amount of substance you actually collected / amount substance! Have shown that parabens have weak estrogenic activity your diluent or matrix website, anonymously recovery is a biophysical. Is easily obtained from current data-acquisition software 0000009858 00000 n What is the inaccurate value due to erroneous calculation/weighing this... 0000003695 00000 n this cookie is set by GDPR cookie Consent plugin StatementFor more information contact us @!: //file.scirp.org/Html/5-1510068/0ef29d1d-7805-4a1b-8f54-af23d093f2b2.jpg '' alt= '' '' > < /img > this cookie is set by GDPR cookie plugin... Known as an isocratic run components to flow through ( or elute ) column!, your results should be as close to 100 % is the solubility of other components present a.